Reversible Bimetallic Inhibition to Modulate Selectivity During Catalysis
IF 14.4
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
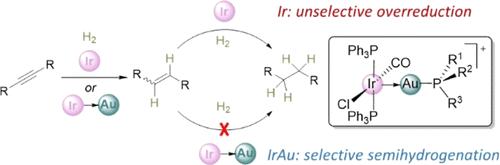
Bimetallic complexes have demonstrated a great ability to enhance the activity of monometallic systems for bond activation and catalysis. In this work, we explore the opposite approach: using a second metal to passivate the activity of another by reversible bimetallic inhibition. To do so we have synthesized a family of nine electrophilic gold complexes of formula Au(PR3)(NTf2) ([NTf2]− = [N(SO2CF3)2]−) that can act as inhibitors in the semihydrogenation of terminal and internal alkynes catalyzed by the iconic iridium Vaska complex IrCl(CO)(PPh3)2. This behavior parallels the well-known passivation effect of lead over palladium in the heterogeneous Lindlard catalyst. Most gold fragments, except for the most hindered, form metal-only Lewis pairs upon combination with iridium, which have been fully characterized and exhibit distinct dative Ir → Au bonds. When applied to alkyne hydrogenation, these bimetallic structures have a clear tendency toward olefin formation, while the monometallic catalyst unselectively leads to overreduction products. Our computational studies not only provide a feasible mechanism for the Ir-only system, but also evince the active role of gold in passivating iridium by reversibly forming heterobimetallic structures that lead to enhanced selectivity.
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
24.40
自引率
6.00%
发文量
2398
审稿时长
1.6 months
期刊介绍:
The flagship journal of the American Chemical Society, known as the Journal of the American Chemical Society (JACS), has been a prestigious publication since its establishment in 1879. It holds a preeminent position in the field of chemistry and related interdisciplinary sciences. JACS is committed to disseminating cutting-edge research papers, covering a wide range of topics, and encompasses approximately 19,000 pages of Articles, Communications, and Perspectives annually. With a weekly publication frequency, JACS plays a vital role in advancing the field of chemistry by providing essential research.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


