Quantification of vehicular versus uncorrelated Li+–solvent transport in highly concentrated electrolytes via solvent-related Onsager coefficients
IF 2.9
3区 化学
Q3 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
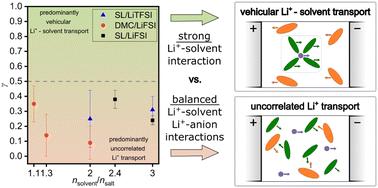
Highly concentrated salt solutions are promising electrolytes for battery applications due to their low flammability, their high thermal stability, and their good compatibility with electrode materials. Understanding transport processes in highly concentrated electrolytes is a challenging task, since strong ion–ion and ion–solvent interactions lead to highly correlated movements on the microscopic scale. Here, we use an experimental overdetermination method to obtain accurate Onsager transport coefficients for concentrated binary electrolytes composed of either sulfolane (SL) or dimethyl carbonate (DMC) as solvent and either LiTFSI or LiFSI as salt. NMR-based electrophoretic mobilities demonstrate that volume conservation applies as a governing constraint for the transport. This fact allows to calculate the Onsager coefficients σ+0, σ−0 and σ00 related to the solvent. A parameter γ is then defined, which is a measure for the relevance of a vehicular Li+–solvent transport mechanism. We analyze the influence of the salt anion and of the solvent on dynamic correlations and transport mechanisms. In the case of the sulfolane-based electrolytes, the γ parameter reaches values up to 0.38, indicating that Li+–sulfolane interactions are stronger than Li+–anion interactions and that vehicular Li+–sulfolane transport plays a significant role. In the case of DMC-based electrolytes, the γ parameter is close to zero, suggesting balanced Li+–DMC vs. Li+–anion interactions and virtually uncorrelated movements of Li+ ions and DMC molecules.
求助全文
约1分钟内获得全文
求助全文
来源期刊

Physical Chemistry Chemical Physics
化学-物理:原子、分子和化学物理
CiteScore
5.50
自引率
9.10%
发文量
2675
审稿时长
2.0 months
期刊介绍:
Physical Chemistry Chemical Physics (PCCP) is an international journal co-owned by 19 physical chemistry and physics societies from around the world. This journal publishes original, cutting-edge research in physical chemistry, chemical physics and biophysical chemistry. To be suitable for publication in PCCP, articles must include significant innovation and/or insight into physical chemistry; this is the most important criterion that reviewers and Editors will judge against when evaluating submissions.
The journal has a broad scope and welcomes contributions spanning experiment, theory, computation and data science. Topical coverage includes spectroscopy, dynamics, kinetics, statistical mechanics, thermodynamics, electrochemistry, catalysis, surface science, quantum mechanics, quantum computing and machine learning. Interdisciplinary research areas such as polymers and soft matter, materials, nanoscience, energy, surfaces/interfaces, and biophysical chemistry are welcomed if they demonstrate significant innovation and/or insight into physical chemistry. Joined experimental/theoretical studies are particularly appreciated when complementary and based on up-to-date approaches.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


