Influence of Dissolved Iron in Solution on MgO Hydroxylation and Carbonation
IF 3.2
3区 化学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
MgO (periclase) is a promising material for direct air capture of CO2 using a mineral looping process, but it is unknown how impurities in the environment will affect the CO2 uptake and hence process economics. Here, we investigated the effects of dissolved iron on the extents of MgO hydroxylation and subsequent carbonation reactions to determine if this has a beneficial or detrimental effect. On single-crystal MgO, dissolved iron prevented hydration of MgO to Mg(OH)2 (brucite) and instead formed a shell of lepidocrocite (γ-FeOOH). This did not passivate the MgO as dissolution below the shell was observed. During hydroxylation of MgO powders in the presence of dissolved iron, formation of brucite containing Fe(II) was observed. In addition, formation of nanoscale iron oxides containing Fe(III) was observed using magnetometry and Mössbauer spectroscopy. Subsequent carbonation experiments showed increased carbonation of MgO hydroxylated in the presence of iron. Our results indicate that the presence of dissolved solute impurities during hydroxylation may be beneficial for carbonation of hydroxylated MgO.
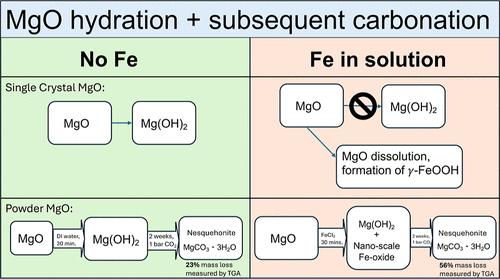
溶液中溶解铁对氧化镁羟基化和碳酸化的影响
MgO(方长石)是一种很有前途的材料,可以使用矿物循环工艺直接捕捉空气中的二氧化碳,但目前尚不清楚环境中的杂质如何影响二氧化碳的吸收,从而影响过程的经济性。在这里,我们研究了溶解的铁对氧化镁羟基化程度和随后的碳化反应的影响,以确定这是有益的还是有害的影响。在单晶MgO上,溶解的铁阻止了MgO与Mg(OH)2(水镁石)的水化作用,形成了一个鳞片石(γ-FeOOH)的壳层。这并没有钝化氧化镁,因为在壳下观察到溶解。在溶解的铁存在下,氧化镁粉羟基化过程中,观察到形成含铁(II)的水镁石。此外,利用磁强计和Mössbauer光谱法观察了含Fe(III)的纳米级氧化铁的形成。随后的碳化实验表明,在铁的存在下,氧化镁羟基化的碳化作用增加。我们的研究结果表明,羟基化过程中溶解的溶质杂质的存在可能有利于羟基化MgO的碳化。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

The Journal of Physical Chemistry C
化学-材料科学:综合
CiteScore
6.50
自引率
8.10%
发文量
2047
审稿时长
1.8 months
期刊介绍:
The Journal of Physical Chemistry A/B/C is devoted to reporting new and original experimental and theoretical basic research of interest to physical chemists, biophysical chemists, and chemical physicists.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


