Lanthanum-Promoted Electrocatalyst for the Oxygen Evolution Reaction: Unique Catalyst or Oxide Deconstruction?
IF 14.4
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
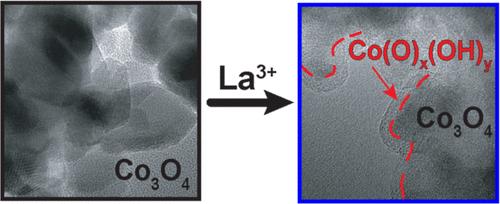
A conventional performance metric for electrocatalysts that promote the oxygen evolution reaction (OER) is the current density at a given overpotential. However, the assumption that increased current density at lower overpotentials indicates superior catalyst design is precarious for OER catalysts in the working environment, as the crystalline lattice is prone to deconstruction and amorphization, thus greatly increasing the concentration of catalytic active sites. We show this to be the case for La3+ incorporation into Co3O4. Powder X-ray diffraction (PXRD), Raman spectroscopy and extended X-ray absorption fine structure (EXAFS) reveal smaller domain sizes with decreased long-range order and increased amorphization for La-modified Co3O4. This lattice deconstruction is exacerbated under the conditions of OER as indicated by operando spectroscopies. The overpotential for OER decreases with increasing La3+ concentration, with maximum activity achieved at 17% La incorporation. HRTEM images and electron diffraction patterns clearly show the formation of an amorphous overlayer during OER catalysis that is accelerated with La3+ addition. O 1s XPS spectra after OER show the loss of lattice-oxide and an increase in peak intensities associated with hydroxylated or defective O-atom environments, consistent with Co(O)x(OH)y species in an amorphous overlayer. Our results suggest that improved catalytic activity of oxides incorporated with La3+ ions (and likely other metal ions) is due to an increase in the number of terminal octahedral Co(O)x(OH)y edge sites upon Co3O4 lattice deconstruction, rather than enhanced intrinsic catalysis.
镧促进的析氧电催化剂:独特的催化剂还是氧化物的解构?
促进氧进化反应(OER)的电催化剂的传统性能指标是给定过电位下的电流密度。然而,假定在较低过电位下电流密度增加表明催化剂设计优良,这对于工作环境中的 OER 催化剂来说是不稳定的,因为晶格容易解构和非晶化,从而大大增加催化活性位点的浓度。我们在将 La3+ 加入 Co3O4 时证明了这一点。粉末 X 射线衍射 (PXRD)、拉曼光谱和扩展 X 射线吸收精细结构 (EXAFS) 显示,La 改性 Co3O4 的畴尺寸变小,长程有序性降低,非晶化增加。操作光谱显示,在 OER 条件下,晶格解构加剧。OER 的过电位随着 La3+ 浓度的增加而降低,当 La 的掺入量达到 17% 时,活性达到最大。HRTEM 图像和电子衍射图清楚地显示了在 OER 催化过程中无定形覆盖层的形成,La3+ 的加入加速了无定形覆盖层的形成。OER 后的 O 1s XPS 光谱显示,晶格氧化物消失,与羟基化或缺陷 O 原子环境相关的峰强度增加,这与无定形覆盖层中的 Co(O)x(OH)y 物种一致。我们的研究结果表明,加入 La3+ 离子(也可能是其他金属离子)的氧化物催化活性的提高是由于 Co3O4 晶格解构后,末端八面体 Co(O)x(OH)y 边缘位点数量的增加,而不是内在催化作用的增强。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
24.40
自引率
6.00%
发文量
2398
审稿时长
1.6 months
期刊介绍:
The flagship journal of the American Chemical Society, known as the Journal of the American Chemical Society (JACS), has been a prestigious publication since its establishment in 1879. It holds a preeminent position in the field of chemistry and related interdisciplinary sciences. JACS is committed to disseminating cutting-edge research papers, covering a wide range of topics, and encompasses approximately 19,000 pages of Articles, Communications, and Perspectives annually. With a weekly publication frequency, JACS plays a vital role in advancing the field of chemistry by providing essential research.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


