Controllable Active Intermediate in CO2 Hydrogenation Enabling Highly Selective N,N-Dimethylformamide Synthesis via N-Formylation
IF 15.6
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
N,N-Dimethylformamide (DMF) is a widely used solvent, and its green and low-carbon synthesis methods are in high demand. Herein, we report a new approach for DMF synthesis using a continuous flow reaction system with a fixed-bed reactor and a ZnO-TiO2 solid solution catalyst. This catalyst effectively utilizes CO2, H2, and dimethylamine (DMA) as feedstocks, demonstrating performance with 99% DMF selectivity and single-pass DMA conversion approaching thermodynamic equilibrium. Moreover, the catalyst demonstrates good stability, with no signs of deactivation over 1000 h of continuous operation. The key to superior activity lies in the synergetic effect of the Zn and Ti sites, which facilitates the formation of active formate species. These species act as crucial intermediates, reacting with DMA to produce DMF. Importantly, the slow hydrogenation kinetics of the formate species prevent the formation of CH2O* species, thereby suppressing the formation of the undesired byproduct, trimethylamine. This work underscores the potential of kinetically controlling active intermediates in CO2 hydrogenation to prepare high-value-added chemicals by coupling them to platform molecules. It presents a promising strategy for the efficient utilization of CO2 resources and offers a valuable solution for large-scale DMF synthesis.
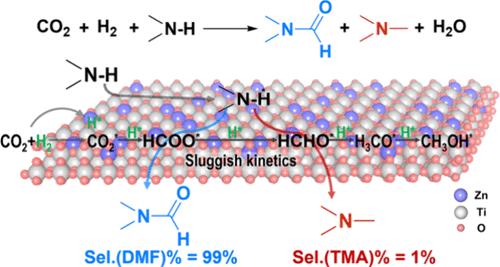
CO2加氢过程中可控的活性中间体通过N-甲酰化合成高选择性N,N-二甲基甲酰胺
N,N-二甲基甲酰胺(DMF)是一种应用广泛的溶剂,其绿色低碳的合成方法需求量很大。本文报道了一种采用固定床反应器和ZnO-TiO2固溶体催化剂的连续流动反应系统合成DMF的新方法。该催化剂有效地利用CO2、H2和二甲胺(DMA)作为原料,表现出99%的DMF选择性和接近热力学平衡的单道DMA转化性能。此外,该催化剂表现出良好的稳定性,在连续运行1000小时以上没有失活迹象。活性优异的关键在于Zn和Ti位点的协同作用,促进了活性甲酸物的形成。这些物种作为关键的中间体,与DMA反应产生DMF。重要的是,甲酸酯的缓慢氢化动力学阻止了CH2O*的形成,从而抑制了不需要的副产物三甲胺的形成。这项工作强调了在CO2加氢过程中通过动力学控制活性中间体与平台分子偶联来制备高附加值化学品的潜力。它为二氧化碳资源的有效利用提供了一个有前途的策略,并为大规模合成DMF提供了有价值的解决方案。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
24.40
自引率
6.00%
发文量
2398
审稿时长
1.6 months
期刊介绍:
The flagship journal of the American Chemical Society, known as the Journal of the American Chemical Society (JACS), has been a prestigious publication since its establishment in 1879. It holds a preeminent position in the field of chemistry and related interdisciplinary sciences. JACS is committed to disseminating cutting-edge research papers, covering a wide range of topics, and encompasses approximately 19,000 pages of Articles, Communications, and Perspectives annually. With a weekly publication frequency, JACS plays a vital role in advancing the field of chemistry by providing essential research.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


