Total Syntheses of Scabrolide B, Ineleganolide, and Related Norcembranoids
IF 14.4
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
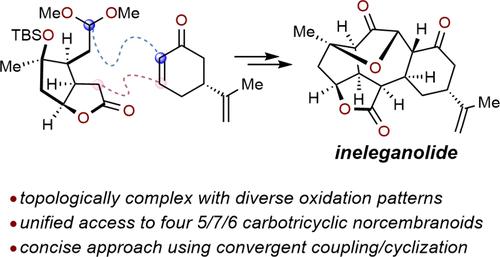
Concise total syntheses of several 5/7/6 norcembranoids, including ineleganolide, scabrolide B, sinuscalide C, and fragilolide A have been achieved through a fragment coupling/ring closure approach. The central seven-membered ring was forged through sequential Mukaiyama–Michael/aldol reactions using norcarvone and a decorated bicyclic lactone incorporating a latent electrophile. Subsequent manipulations installed the reactive enedione motif and delivered scabrolide B in 11 steps from a chiral pool-derived enone. Finally, ineleganolide, sinuscalide C, and fragilolide A were each accessed in one additional step.
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
24.40
自引率
6.00%
发文量
2398
审稿时长
1.6 months
期刊介绍:
The flagship journal of the American Chemical Society, known as the Journal of the American Chemical Society (JACS), has been a prestigious publication since its establishment in 1879. It holds a preeminent position in the field of chemistry and related interdisciplinary sciences. JACS is committed to disseminating cutting-edge research papers, covering a wide range of topics, and encompasses approximately 19,000 pages of Articles, Communications, and Perspectives annually. With a weekly publication frequency, JACS plays a vital role in advancing the field of chemistry by providing essential research.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


