A Self-Assembled Nano-Molecular Glue (Nano-mGlu) Enables GSH/H2O2-Triggered Targeted Protein Degradation in Cancer Therapy
IF 15.6
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
Molecular glues are promising protein-degrading agents that hold great therapeutic potential but face significant challenges in rational design, effective synthesis, and precise targeting of tumor sites. In this study, we first overcame some of these limitations by introducing a fumarate-based molecular glue handle onto specific ligands of therapeutic kinases (TBK1, FGFR, and Bcr-Abl), resulting in the effective degradation of these important cancer targets. Despite the broad applicability of the strategy, we unexpectedly discovered potent and widespread cytotoxicity across various cell lines, including noncancerous ones, rendering it less effective in cancer therapy. To address this critical issue, we next developed a self-assembled nanoparticle-based molecular glue (nano-mGlu) based on one of the newly discovered Bcr-Abl-degrading molecular glues (H1-mGlu). We showed that the resulting nano-mGlu (named Cle-NP) was able to release H1-mGlu in vitro in the presence of a high concentration of GSH or H2O2 (commonly found in the tumor microenvironment). Subsequent in vivo antitumor studies with a K562-xenografted mouse model indicated that Cle-NP was highly effective in tumor-specific degradation of endogenous Bcr-Abl expressed in K562 cells, leading to eventual tumor regression while maintaining good biosafety profiles. With key advantages of generality in molecular glue design, targeted delivery (e.g., H1-mGlu), potent antitumor activity partially induced by target-specific degradation, and minimized collateral damage to healthy tissues, our self-assembled nano-mGlu strategy thus provides a novel approach that might hold a significant promise for effective and personalized cancer therapy.
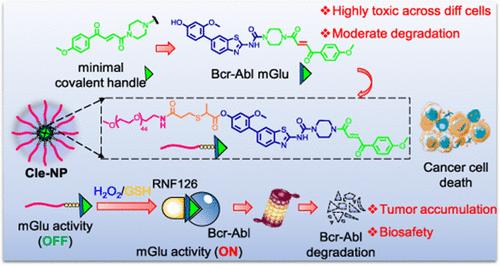
一种自组装纳米分子胶(Nano-mGlu)使GSH/ h2o2触发的靶向蛋白质降解在癌症治疗中成为可能
分子胶是一种很有前途的蛋白质降解剂,具有巨大的治疗潜力,但在合理设计、有效合成和精确靶向肿瘤部位方面面临重大挑战。在这项研究中,我们首先通过将富马酸基分子胶处理引入治疗性激酶(TBK1, FGFR和Bcr-Abl)的特定配体上,克服了这些局限性,从而有效降解了这些重要的癌症靶点。尽管该策略具有广泛的适用性,但我们意外地发现,该策略在各种细胞系(包括非癌细胞系)中具有强大而广泛的细胞毒性,使其在癌症治疗中效果较差。为了解决这一关键问题,我们下一步基于新发现的bcr - abl降解分子胶(H1-mGlu)开发了一种自组装的纳米颗粒分子胶(nano-mGlu)。我们发现,得到的纳米mglu(命名为Cle-NP)能够在体外高浓度GSH或H2O2(常见于肿瘤微环境)存在的情况下释放H1-mGlu。随后的K562异种移植小鼠模型的体内抗肿瘤研究表明,Cle-NP在K562细胞中表达的内源性Bcr-Abl的肿瘤特异性降解方面非常有效,最终导致肿瘤消退,同时保持良好的生物安全性。凭借分子胶设计的通用性、靶向递送(例如H1-mGlu)、部分由靶标特异性降解诱导的有效抗肿瘤活性以及对健康组织的附带损伤最小化等关键优势,我们的自组装纳米mglu策略为有效和个性化的癌症治疗提供了一种新的方法。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
24.40
自引率
6.00%
发文量
2398
审稿时长
1.6 months
期刊介绍:
The flagship journal of the American Chemical Society, known as the Journal of the American Chemical Society (JACS), has been a prestigious publication since its establishment in 1879. It holds a preeminent position in the field of chemistry and related interdisciplinary sciences. JACS is committed to disseminating cutting-edge research papers, covering a wide range of topics, and encompasses approximately 19,000 pages of Articles, Communications, and Perspectives annually. With a weekly publication frequency, JACS plays a vital role in advancing the field of chemistry by providing essential research.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


