Two Blatter Radicals Face-to-Face: A Constrained Diradical Architecture
IF 14.4
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
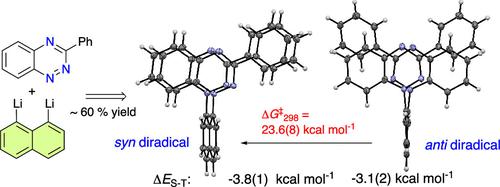
Cofacial arrangement of two Blatter radicals enforced by the peri-naphthalene scaffold represents a new approach to stable diradicals with strong through-space interactions. Two stereoisomers of the naphthalene-diradicals, anti and syn, are investigated by XRD, VT-EPR, UV–vis, electrochemical, kinetic, and DFT methods. In solutions, both stereoisomers exist as open-shell singlets with ΔES-T = −3.1 and −3.8 kcal mol–1, respectively. The anti isomer was resolved into enantiomers and converts to syn with ΔG‡298 = 23.6(8) kcal mol–1.
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
24.40
自引率
6.00%
发文量
2398
审稿时长
1.6 months
期刊介绍:
The flagship journal of the American Chemical Society, known as the Journal of the American Chemical Society (JACS), has been a prestigious publication since its establishment in 1879. It holds a preeminent position in the field of chemistry and related interdisciplinary sciences. JACS is committed to disseminating cutting-edge research papers, covering a wide range of topics, and encompasses approximately 19,000 pages of Articles, Communications, and Perspectives annually. With a weekly publication frequency, JACS plays a vital role in advancing the field of chemistry by providing essential research.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


