Metabolic Blockade-Based Genome Mining of Saccharopolyspora erythraea SCSIO 07745: Discovery and Biosynthetic Pathway of Aminoquinolinone Alkaloids Bearing 6/6/5 Tricyclic and 6/6/6/5 Tetracyclic Scaffolds
IF 4.9
1区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
Abstract
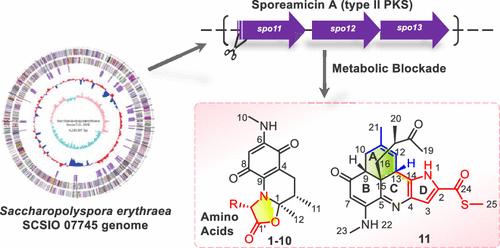
Metabolic blockade-based genome mining of the marine sediment-derived Saccharopolyspora erythraea SCSIO 07745 led to the discovery of 11 novel aminoquinolinone alkaloids, oxazoquinolinones A–J (1–10), characterized by an oxazolidone[3,2-α]quinoline-5,8-dione scaffold, and oxazoquinolinone K (11), featuring an unprecedented fused 6/6/6/5 tetracyclic core ring system. Additionally, 5 new biosynthetic intermediates or shunt products (12–16) and a known metabolite sannanine (17) were identified. Their structures were elucidated by extensive spectroscopic analyses and a comparison of electronic circular dichroism and single-crystal X-ray diffraction. On the basis of the functional gene analyses and structures of the intermediates or shunt products, plausible biosynthetic pathways for compounds 1–17 were proposed. Additionally, oxazoquinolinone K (11) obviously inhibited cell invasion of human glioma cell line LN229 cells at 10 μM.
求助全文
约1分钟内获得全文
求助全文
来源期刊

Organic Letters
化学-有机化学
CiteScore
9.30
自引率
11.50%
发文量
1607
审稿时长
1.5 months
期刊介绍:
Organic Letters invites original reports of fundamental research in all branches of the theory and practice of organic, physical organic, organometallic,medicinal, and bioorganic chemistry. Organic Letters provides rapid disclosure of the key elements of significant studies that are of interest to a large portion of the organic community. In selecting manuscripts for publication, the Editors place emphasis on the originality, quality and wide interest of the work. Authors should provide enough background information to place the new disclosure in context and to justify the rapid publication format. Back-to-back Letters will be considered. Full details should be reserved for an Article, which should appear in due course.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


