Impact of Anions and Water Content on [Li–Al] Layered Double-Hydroxide Stability
IF 3.3
3区 化学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
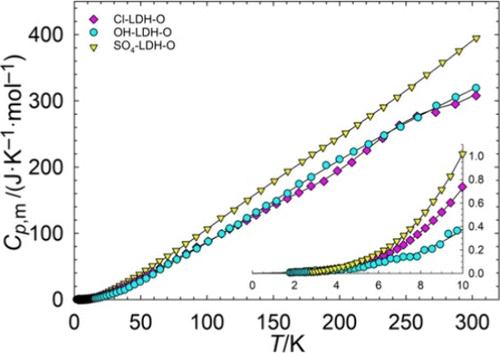
[Li–Al] layered double hydroxides (LDHs) are compounds with potential as sorbents for lithium extraction from brine solutions. In this work, heat capacities were measured from approximately 2.5 to 300 K for six [Li–Al] LDHs with differing anions (Cl–, OH–, and SO42–) and water content (denoted A for air-dried and O for oven-dried). These measurements were used to calculate the standard entropy at 298.15 K, and the results were combined with previously performed enthalpy measurements to calculate Gibbs energies of formation from the binary compounds. The calculated order of stability based on Gibbs energies of formation was Cl-LDH-O > OH-LDH-O > Cl-LDH-A > SO4-LDH-O > SO4-LDH-A > OH-LDH-A. Results support previous findings that higher water content generally raises the Gibbs energy of the LDH.
阴离子和水含量对[Li-Al]层状双氢氧化物稳定性的影响
[锂-铝]层状双氢氧化物(LDHs)是一种具有从盐水溶液中提取锂的吸附剂潜力的化合物。在这项研究中,我们测量了六种具有不同阴离子(Cl-、OH- 和 SO42-)和含水量的[Li-Al] 层状双氢氧化物(空气干燥表示为 A,烘箱干燥表示为 O)在约 2.5 至 300 K 范围内的热容量。这些测量结果用于计算 298.15 K 时的标准熵,并将结果与之前进行的焓测量结果相结合,计算二元化合物形成的吉布斯能。根据形成吉布斯能计算出的稳定性顺序为:Cl-LDH-O > OH-LDH-O > Cl-LDH-A > SO4-LDH-O > SO4-LDH-A > OH-LDH-A。结果支持了之前的发现,即较高的水含量通常会提高 LDH 的吉布斯能。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

The Journal of Physical Chemistry C
化学-材料科学:综合
CiteScore
6.50
自引率
8.10%
发文量
2047
审稿时长
1.8 months
期刊介绍:
The Journal of Physical Chemistry A/B/C is devoted to reporting new and original experimental and theoretical basic research of interest to physical chemists, biophysical chemists, and chemical physicists.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


