Effect of Protecting Groups and Activating Conditions on 3-Deoxy-d-glycero-d-galacto-2-nonulosonic Acid (Kdn) Glycosylation: Stereoselective Synthesis of α- and β-Kdn Glycosides
IF 4.9
1区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
Abstract
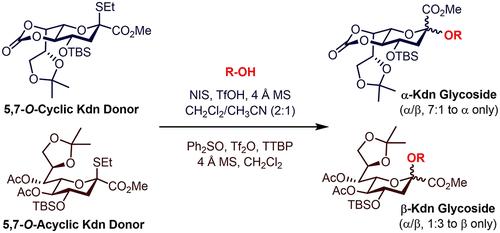
Kdn is a common member of the sialic acid family. Carbohydrates containing Kdn residues are widely distributed in nature and embody important biological information. However, the methods for synthesizing Kdn glycosides are limited, which restricts their biological study. In this paper, we developed efficient α- and β-stereoselective Kdn glycosylation methods by employing differentially protected Kdn thioglycoside donors under their respective activating protocols. The 5,7-O-carbonate fused Kdn thioglycoside 1a could be promoted with NIS/TfOH (cat.) in CH2Cl2/CH3CN (2:1) to afford Kdn glycosides with excellent α-selectivity in high yields. Meanwhile, based on the Ph2SO/Tf2O preactivation strategy, the nonfused Kdn thioglycoside 1b behaved as a high-yielding and β-selective donor to couple with various carbohydrate alcohols, leading to formation of β-Kdn glycosides. The synthetic utility of these newly developed glycosyl donors has been demonstrated by the stereoselective and straightforward assembly of two natural Kdn-containing oligosaccharides.
求助全文
约1分钟内获得全文
求助全文
来源期刊

Organic Letters
化学-有机化学
CiteScore
9.30
自引率
11.50%
发文量
1607
审稿时长
1.5 months
期刊介绍:
Organic Letters invites original reports of fundamental research in all branches of the theory and practice of organic, physical organic, organometallic,medicinal, and bioorganic chemistry. Organic Letters provides rapid disclosure of the key elements of significant studies that are of interest to a large portion of the organic community. In selecting manuscripts for publication, the Editors place emphasis on the originality, quality and wide interest of the work. Authors should provide enough background information to place the new disclosure in context and to justify the rapid publication format. Back-to-back Letters will be considered. Full details should be reserved for an Article, which should appear in due course.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


