Unusually large oxygen non-stoichiometry and defect thermodynamics in Sr4Mn2–xFe1+xO10–δ Ruddlesden-Popper layered oxides
IF 8.3
1区 材料科学
Q1 MATERIALS SCIENCE, MULTIDISCIPLINARY
引用次数: 0
Abstract
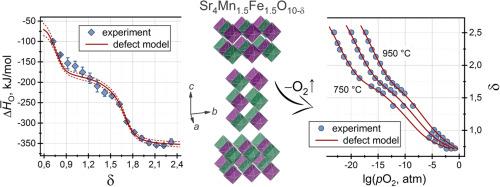
The Ruddlesden-Popper oxides Sr4Mn2–xFe1+xO10–δ (x = 0, 0.5, and 1.0) with the layered tetragonal structure (SG I4/mmm) were synthesized at the heating of organometallic precursors to 1400 °C in air. The Rietveld refinement of the X-ray diffraction patterns indicates a disordered arrangement of Mn and Fe atoms in the crystal. The results of the electron density functional-based calculations of the total energy show noticeably larger oxygen vacancy formation enthalpy for OO2 positions in the structural rock salt layers of strontium oxide compared to the apical OO1 and equatorial OO3 and OO4 positions in perovskite slabs. The coulometric titration measurements show that the Sr4Mn2–xFe1+xO10–δ phases can endure harsh reducing conditions, staying stable even when up to two oxygen atoms are taken away from the structure. Simulations of equilibrium oxygen content data reveal that oxygen exchange is coupled with electron excitation processes involving d-cations and intrinsic anion disorder. It is suggested that this disorder may be caused by unequal vacancy formation energies at crystallographically different oxygen sites. The enthalpy and entropy changes in the defect formation reactions were derived from the fitting of the experimental plots for oxygen non-stoichiometry. The obtained thermodynamic parameters were applied to determine how the concentrations of electronic and ionic defects depend on variations in temperature and partial pressure of oxygen. The consistency of the thermodynamic analysis is supported by a good coincidence of the calculated and experimental plots of the partial thermodynamic functions of labile oxygen in Sr4Mn2-xFe1+xO10–δ.
求助全文
约1分钟内获得全文
求助全文
来源期刊

Acta Materialia
工程技术-材料科学:综合
CiteScore
16.10
自引率
8.50%
发文量
801
审稿时长
53 days
期刊介绍:
Acta Materialia serves as a platform for publishing full-length, original papers and commissioned overviews that contribute to a profound understanding of the correlation between the processing, structure, and properties of inorganic materials. The journal seeks papers with high impact potential or those that significantly propel the field forward. The scope includes the atomic and molecular arrangements, chemical and electronic structures, and microstructure of materials, focusing on their mechanical or functional behavior across all length scales, including nanostructures.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


