A Double-Blind, Placebo-Controlled Study of Adjunctive Topiramate in Adolescents With Co-Occurring Bipolar and Cannabis Use Disorders
引用次数: 0
Abstract
Objective
To evaluate the efficacy of adjunctive topiramate (TPM) for the treatment of cannabis use disorder in adolescents with bipolar I disorder.
Method
We conducted a 16-week, double-blind, randomized, placebo-controlled investigation of quetiapine plus TPM (median dose = 208 mg) vs quetiapine plus placebo in adolescents with bipolar I and cannabis use disorder. All subjects participated in a Motivational Interview and Compliance Enhancement Therapy. The primary outcome measure was change in weekly cannabis use over a 16-week treatment period using the Timeline Followback. The secondary outcome measure was the baseline-to-endpoint total score change in the Young Mania Rating Scale (YMRS).
Results
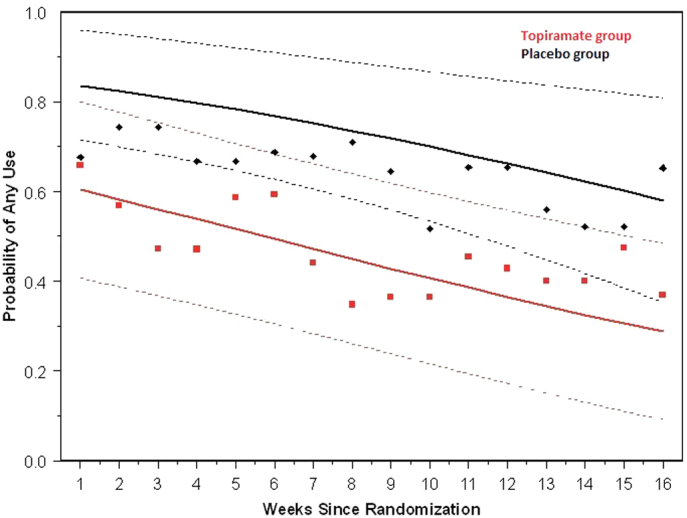
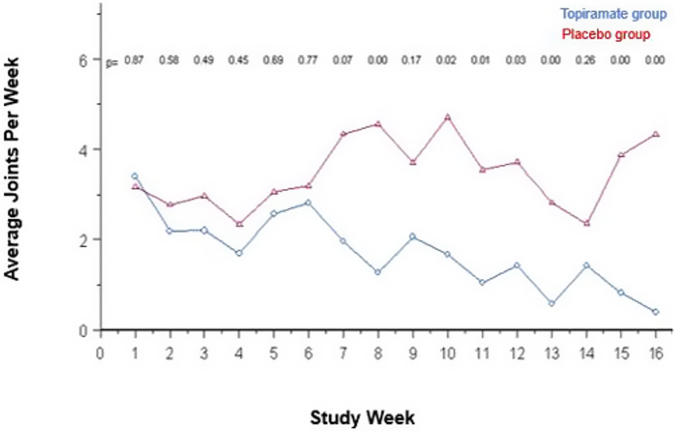
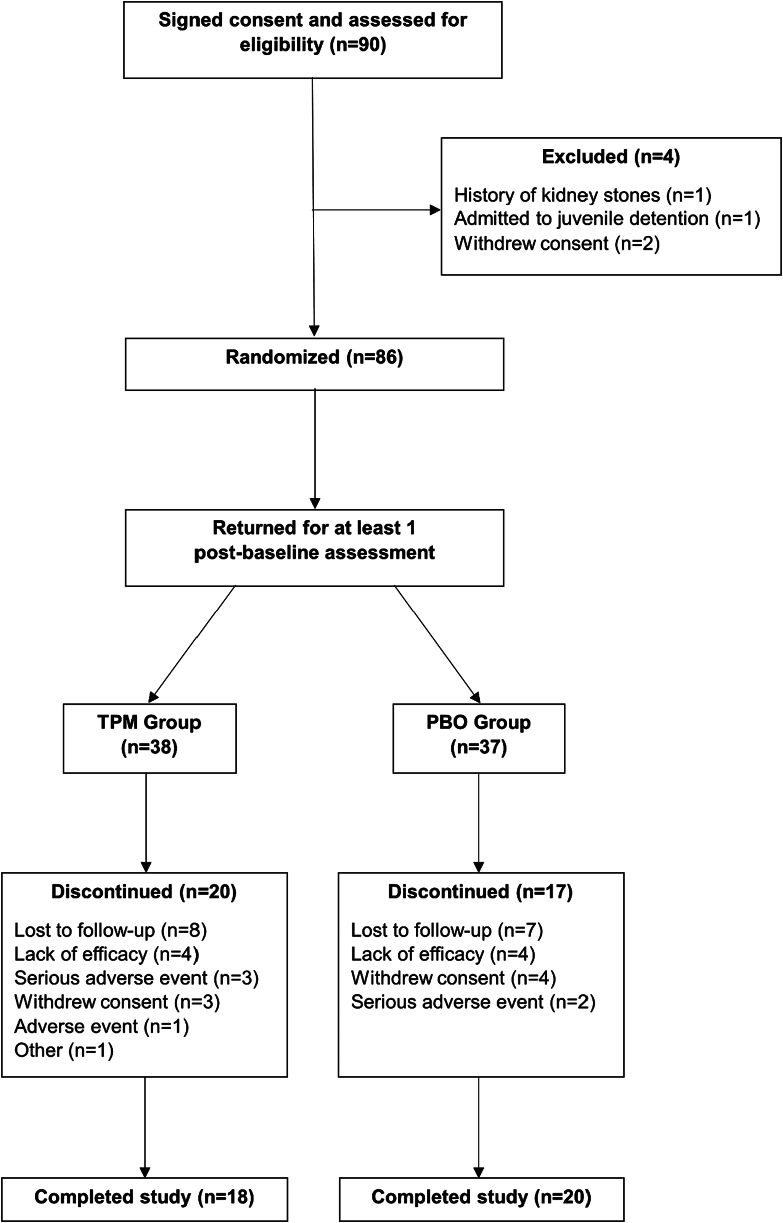
A total of 75 participants completed at least 1 post-baseline assessment (TPM = 38, placebo = 37). There was a significant time-by-treatment effect over the 16-week treatment period, with baseline-adjusted treatment differences in favor of the TPM group over time (p < .001). Although there was no difference in baseline-to-endpoint YMRS total score change between groups (p = .342), there was as significant decline in YMRS total score within both groups (p < .0001). There was a significant positive effect for alcohol use (p < .001) and nicotine use (p = .033) in the TPM group. More participants in the TPM group experienced appetite decrease (p = .032) and excitement (p = .025). Participants in the placebo group experienced greater weight gain (p = .010).
Conclusion
Treatment with TPM adjunctive to quetiapine and a Motivational Interview and Compliance Enhancement Therapy is associated with a greater decrease in cannabis use and less weight gain. TPM is a well-tolerated and efficacious treatment for cannabis use disorder in adolescents with bipolar I disorder.
Plain language summary
Cannabis is the most commonly misused drug in adolescents with bipolar disorder, and there is limited prior research supporting the addition of topiramate to motivational enhancement for the treatment of cannabis use disorder. In this study, 75 adolescents and young adults, aged 12-21, with bipolar I disorder participated in a 16-week, double-blind, randomized, placebo-controlled trial of quetiapine plus topiramate versus quetiapine plus placebo treatment for cannabis use disorder. All subjects participated in a Motivational Interview and Compliance Enhancement Therapy. The group receiving topiramate had a greater decrease in the use of cannabis, alcohol, and nicotine, as well as less weight gain. These results suggest that adjunctive topiramate to quetiapine and motivational interview may be helpful in the treatment of cannabis use disorder in adolescents and young adults with bipolar 1 disorder.
Clinical trial registration information
Efficacy Study of Quetiapine Plus Topiramate for Reducing Cannabis Consumption and Bipolar Mania; https://clinicaltrials.gov/; NCT00393978.
Diversity & Inclusion Statement
We worked to ensure sex and gender balance in the recruitment of human participants. We worked to ensure race, ethnic, and/or other types of diversity in the recruitment of human participants. We worked to ensure that the study questionnaires were prepared in an inclusive way. One or more of the authors of this paper self-identifies as a member of one or more historically underrepresented racial and/or ethnic groups in science.
一项双盲、安慰剂对照研究:托吡酯辅助治疗青少年双相情感障碍和大麻使用障碍。
目的:评价托吡酯(TPM)辅助治疗青少年双相I型障碍大麻使用障碍的疗效。方法:我们对患有双相I型和大麻使用障碍的青少年进行了为期16周的双盲、随机、安慰剂对照研究,比较喹硫平加TPM(中位剂量= 208 mg)与喹硫平加安慰剂。所有受试者均参与动机性访谈和依从性增强治疗。主要结果测量是在16周的治疗期间使用时间线随访的每周大麻使用量的变化。次要结果测量是青年躁狂症评定量表(YMRS)的基线到终点总得分变化。结果:共有75名参与者完成了至少1次基线后评估(TPM = 38,安慰剂= 37)。在16周的治疗期间,有显著的治疗时间效应,随着时间的推移,经过基线调整的治疗差异有利于TPM组(p < 0.001)。虽然两组间基线至终点的YMRS总分变化无差异(p = .342),但两组内YMRS总分均有显著下降(p < .0001)。在TPM组中,酒精使用(p < .001)和尼古丁使用(p = .033)有显著的积极作用。TPM组更多的参与者出现食欲下降(p = 0.032)和兴奋(p = 0.025)。安慰剂组的参与者体重增加更大(p = 0.010)。结论:用TPM辅助喹硫平、动机性访谈和依从性增强治疗与大麻使用的更大减少和更少的体重增加有关。TPM是一种耐受性良好且有效的治疗青少年双相I型障碍大麻使用障碍。临床试验注册信息:喹硫平联合托吡酯减少大麻消费和双相躁狂的疗效研究;https://clinicaltrials.gov/;NCT00393978。多样性和包容性声明:我们努力确保招募人类参与者时的性别和性别平衡。我们努力确保招募人类参与者的种族、民族和/或其他类型的多样性。我们努力确保研究问卷的编制具有包容性。本文的一位或多位作者自认为是科学中一个或多个历史上未被充分代表的种族和/或族裔群体的成员。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


