Hydration Processes of the Proton-Conducting Hexagonal Perovskites Ba7In6–xYxAl2O19
IF 3.3
3区 化学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
The crystal structure, TG-MS, IR, and hydration thermodynamic parameters of the inherently oxygen-deficient Ba7In6–xYxAl2O19 system have been investigated. X-ray diffraction analysis showed that the Ba7In6–xYxAl2O19 (0 ≤ x ≤ 0.25) are hexagonal perovskites belonging to the P63/mmc space group. The hydrated materials exhibited the same structure with larger cell parameters. IR spectroscopy of the hydrated specimens confirmed the existence of three types of hydroxyl groups involved in different hydrogen bonds. The dissolution of water was measured by using thermogravimetric (TG) analysis. The TG measurements showed the water uptake growth with increase of x. Dehydration upon heating is a three-stage process, namely, two close effects in the temperature range 200–600 °C and a small high-temperature effect near 800 °C. A defect chemical model was developed to derive hydration thermodynamic parameters based on TG data. A thermodynamic approach to the hydration process showed that the increase in the Y3+ content was accompanied by a decrease in the enthalpy of hydration due to the enlargement in the basicity of the phases. The strategy of introducing an isovalent dopant with a larger size and lower electronegativity turned out to be favorable in terms of increasing the proton concentration and decreasing the enthalpy of hydration.
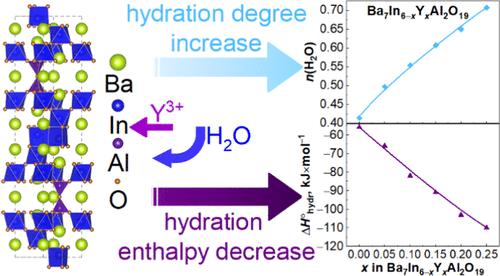
质子导电六方钙钛矿Ba7In6-xYxAl2O19的水化过程
研究了固有缺氧Ba7In6-xYxAl2O19体系的晶体结构、TG-MS、IR和水化热力学参数。x射线衍射分析表明,Ba7In6-xYxAl2O19(0≤x≤0.25)为六方钙钛矿,属于P63/mmc空间基团。水合材料具有相同的结构,但细胞参数较大。水化样品的红外光谱证实了三种类型的羟基参与不同的氢键的存在。用热重法(TG)测定水的溶解度。热重测量表明,随着x的增加,吸水量增加。加热后脱水是一个三个阶段的过程,即在200-600℃范围内有两个密切的影响,在800℃附近有一个小的高温影响。建立了基于热重数据的缺陷化学模型,推导了水化热力学参数。对水化过程的热力学分析表明,随着Y3+含量的增加,由于相的碱度增大,水化焓随之降低。引入尺寸更大、电负性更低的同价掺杂剂有利于提高质子浓度,降低水化焓。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

The Journal of Physical Chemistry C
化学-材料科学:综合
CiteScore
6.50
自引率
8.10%
发文量
2047
审稿时长
1.8 months
期刊介绍:
The Journal of Physical Chemistry A/B/C is devoted to reporting new and original experimental and theoretical basic research of interest to physical chemists, biophysical chemists, and chemical physicists.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


