Effect of Internal Electron Donors on Butadiene Polymerization Catalyzed by the TiCl4/MgCl2 Ziegler–Natta Catalyst
IF 3.2
3区 化学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
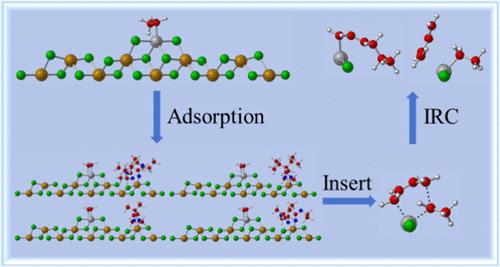
Ziegler–Natta (ZN) catalysts recognized as mainstream and efficient polymerization reaction catalysts are widely used for the polymerization of monolefins and diolefins due to their excellent properties. The addition of electron donors to the catalyst can effectively improve its regularity. In this article, the chelate coordination of the internal electron donors 2,3-dimethyl diethylsuccinate (DES), 2,3-diisopropyl diethylsuccinate (DIS), 2,2-dimethyl-1,3-dimethoxypropane (DMMP), and 2,2-diisopropyl-1,3-dimethoxypropane (DIMP) on the catalyst support was investigated by the density functional theory (DFT). By calculating the activation energy of the reaction before and after the addition of the internal electron donor, the effect of the internal electron donor on the stereoselectivity of the polymerization reaction was analyzed. The results indicate that all four internal electron donors can be stably adsorbed onto the support, forming closer interactions with adjacent active centers. In the butadiene insertion process, the si configuration is more favorable than the re configuration. With the addition of DES, DIS, DMMP, and DIMP, the stereoselectivity of the polymerization reaction increased from 0.7 kcal/mol on the bare active center model to 0.9, 1.2, 0.8, and 1.5 kcal/mol, respectively. Particularly noteworthy is the fact that the addition of DIMP showed the most pronounced improvement in reaction stereoselectivity.
内给电子体对TiCl4/MgCl2 Ziegler-Natta催化剂催化丁二烯聚合的影响
Ziegler-Natta (ZN)催化剂因其优异的性能被广泛应用于单烯烃和二烯烃的聚合反应,是公认的主流高效聚合反应催化剂。在催化剂中加入电子给体可以有效地改善催化剂的规整性。利用密度泛函理论(DFT)研究了内电子给体2,3-二甲基二乙基琥珀酸酯(DES)、2,3-二异丙基二乙基琥珀酸酯(DIS)、2,2-二甲基-1,3-二甲氧基丙烷(DMMP)和2,2-二异丙基1,3-二甲氧基丙烷(DIMP)在催化剂载体上的螯合配位。通过计算加入内电子给体前后反应的活化能,分析了内电子给体对聚合反应立体选择性的影响。结果表明,四种内给电子体都能稳定地吸附在载体上,并与邻近的活性中心形成更紧密的相互作用。在丁二烯插入过程中,si构型比重构型更有利。随着DES、DIS、DMMP和DIMP的加入,聚合反应的立体选择性分别从裸活性中心模型下的0.7 kcal/mol提高到0.9、1.2、0.8和1.5 kcal/mol。特别值得注意的是,DIMP的加入对反应立体选择性的改善最为显著。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

The Journal of Physical Chemistry C
化学-材料科学:综合
CiteScore
6.50
自引率
8.10%
发文量
2047
审稿时长
1.8 months
期刊介绍:
The Journal of Physical Chemistry A/B/C is devoted to reporting new and original experimental and theoretical basic research of interest to physical chemists, biophysical chemists, and chemical physicists.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


