Oxidation of Silicon Carbide with Atomic Oxygen through the Passive-to-Active Transition
IF 3.2
3区 化学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
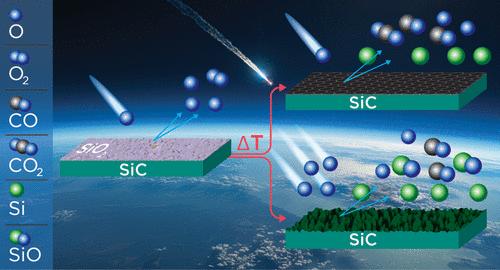
The inelastic and reactive scattering dynamics of O(3P) atoms on a 6H-silicon carbide (SiC) surface were investigated as a function of temperature with a molecular beam-surface scattering technique that employed a rotatable mass spectrometer detector. One set of experiments used a pulsed hyperthermal beam that produced ∼8 km s–1 O atoms, and another set of experiments used a continuous beam that contained O atoms with a nominal velocity of ∼2 km s–1. When exposed to the relatively low-flux, pulsed hyperthermal O-atom beam, a passive oxide layer on the SiC that formed at lower temperatures decomposed as the temperature increased above 1673 K. Instead of entering an active oxidation regime, a graphitic layer formed on the surface and largely protected the underlying SiC from incoming O atoms. Investigation of the oxide decomposition without O-atom bombardment revealed volatile Si and SiO products at the moment that the oxide decomposed, implying that Si sublimation occurs concurrently with oxide decomposition. In the experiment with the continuous (higher flux) O-atom beam, volatile SiO and CO products were observed after the passive oxide decomposed, indicating that active oxidation was reached. The results of this study provide evidence for the importance of Si sublimation within the active oxidation regime. Above the passive-to-active transition temperature, Si sublimation is an ongoing process, and continuous active oxidation will only occur if sufficient oxygen reacts with the surface to produce SiO more quickly than a graphitic layer can form. Thus, accurate models of SiC ablation by atomic oxygen must account for multiple competing mechanisms that depend on the surface temperature and incident O-atom flux.
碳化硅与原子氧通过被动到主动转变的氧化
采用可旋转质谱仪分子束表面散射技术,研究了O(3P)原子在6h型碳化硅(SiC)表面的非弹性和反应散射动力学与温度的关系。一组实验使用了产生约8 km s-1 O原子的脉冲超热束,另一组实验使用了包含O原子的连续束,标称速度为约2 km s-1。当暴露在相对低通量的脉冲高温o原子束下时,当温度高于1673 K时,在较低温度下形成的SiC表面的被动氧化层分解。而不是进入活性氧化状态,石墨层在表面形成,并在很大程度上保护了下面的SiC不受O原子的影响。在没有o原子轰击的情况下对氧化物分解的研究发现,在氧化物分解的同时,挥发性的Si和SiO产物也在分解,这意味着Si升华与氧化物分解同时发生。在连续(高通量)o原子束的实验中,观察到被动氧化物分解后挥发的SiO和CO产物,表明达到了主动氧化。本研究的结果为Si升华在活性氧化机制中的重要性提供了证据。在钝化到活性转变温度以上,Si升华是一个持续的过程,只有当足够的氧气与表面反应以比石墨层形成更快的速度生成SiO时,才会发生持续的活性氧化。因此,原子氧烧蚀SiC的精确模型必须考虑依赖于表面温度和入射o原子通量的多种竞争机制。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

The Journal of Physical Chemistry C
化学-材料科学:综合
CiteScore
6.50
自引率
8.10%
发文量
2047
审稿时长
1.8 months
期刊介绍:
The Journal of Physical Chemistry A/B/C is devoted to reporting new and original experimental and theoretical basic research of interest to physical chemists, biophysical chemists, and chemical physicists.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


