Electroredox N-Heterocyclic Carbene-Catalyzed Enantioselective (3 + 3) Annulation of Enals with 2-Naphthols
IF 4.9
1区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
Abstract
Developing asymmetric transformations using electroredox and N-heterocyclic carbene (NHC)-catalyzed radical pathways is still desirable and challenging. Herein, we report an iodide-promoted β-carbon activation (LUMO-lowering process) of enals via electroredox carbene catalysis coupled with a hydrogen evolution reaction (HER). This strategy offers an environmentally friendly and sustainable route for rapidly assembling synthetically useful chiral naphthopyran-3-one in good to excellent yield and enantioselectivity using traceless electrons as inexpensive and greener oxidants. The mechanistic studies and cyclic voltammetry suggest that the reaction proceeds via direct single electron transfer (SET) of the in situ-generated Breslow intermediate.
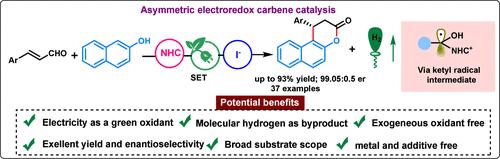
利用电氧化还原和 N-heterocyclic carbene (NHC) 催化的自由基途径开发不对称转化仍然是理想和具有挑战性的。在此,我们报告了一种通过电氧化还原碳烯催化与氢进化反应(HER)相结合的碘化物促进烯醛的 β 碳活化(LUMO 降低过程)的方法。这种策略提供了一种环境友好型和可持续发展的途径,利用无痕电子作为廉价和更环保的氧化剂,快速组装出具有良好甚至卓越产率和对映体选择性的有用手性萘-3-酮。机理研究和循环伏安法表明,该反应是通过原位生成的布雷斯罗中间体的直接单电子转移(SET)进行的。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Organic Letters
化学-有机化学
CiteScore
9.30
自引率
11.50%
发文量
1607
审稿时长
1.5 months
期刊介绍:
Organic Letters invites original reports of fundamental research in all branches of the theory and practice of organic, physical organic, organometallic,medicinal, and bioorganic chemistry. Organic Letters provides rapid disclosure of the key elements of significant studies that are of interest to a large portion of the organic community. In selecting manuscripts for publication, the Editors place emphasis on the originality, quality and wide interest of the work. Authors should provide enough background information to place the new disclosure in context and to justify the rapid publication format. Back-to-back Letters will be considered. Full details should be reserved for an Article, which should appear in due course.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


