Quantitative profiling of human translation initiation reveals elements that potently regulate endogenous and therapeutically modified mRNAs
IF 14.5
1区 生物学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
Abstract
mRNA therapeutics offer a potentially universal strategy for the efficient development and delivery of therapeutic proteins. Current mRNA vaccines include chemically modified nucleotides to reduce cellular immunogenicity. Here, we develop an efficient, high-throughput method to measure human translation initiation on therapeutically modified as well as endogenous RNAs. Using systems-level biochemistry, we quantify ribosome recruitment to tens of thousands of human 5′ untranslated regions (UTRs) including alternative isoforms and identify sequences that mediate 200-fold effects. We observe widespread effects of coding sequences on translation initiation and identify small regulatory elements of 3–6 nucleotides that are sufficient to potently affect translational output. Incorporation of N1-methylpseudouridine (m1Ψ) selectively enhances translation by specific 5′ UTRs that we demonstrate surpass those of current mRNA vaccines. Our approach is broadly applicable to dissecting mechanisms of human translation initiation and engineering more potent therapeutic mRNAs.
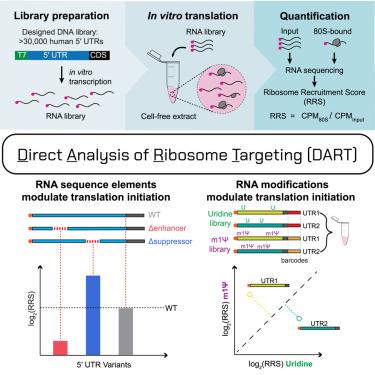
人类翻译起始的定量分析揭示了有效调节内源性和治疗修饰mrna的元素
mRNA疗法为有效开发和递送治疗性蛋白提供了一种潜在的通用策略。目前的mRNA疫苗包括化学修饰的核苷酸以降低细胞免疫原性。在这里,我们开发了一种高效,高通量的方法来测量治疗修饰和内源性rna上的人类翻译起始。利用系统级生物化学,我们量化了核糖体招募到成千上万的人类5 '非翻译区(utr),包括替代异构体,并鉴定了介导200倍效应的序列。我们观察到编码序列对翻译起始的广泛影响,并确定了3-6个核苷酸的小调控元件,足以有效地影响翻译输出。n1 -甲基假尿嘧啶(m1Ψ)的掺入选择性地增强了特定5 ' utr的翻译,我们证明其优于目前的mRNA疫苗。我们的方法广泛适用于解剖人类翻译起始机制和设计更有效的治疗性mrna。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Molecular Cell
生物-生化与分子生物学
CiteScore
26.00
自引率
3.80%
发文量
389
审稿时长
1 months
期刊介绍:
Molecular Cell is a companion to Cell, the leading journal of biology and the highest-impact journal in the world. Launched in December 1997 and published monthly. Molecular Cell is dedicated to publishing cutting-edge research in molecular biology, focusing on fundamental cellular processes. The journal encompasses a wide range of topics, including DNA replication, recombination, and repair; Chromatin biology and genome organization; Transcription; RNA processing and decay; Non-coding RNA function; Translation; Protein folding, modification, and quality control; Signal transduction pathways; Cell cycle and checkpoints; Cell death; Autophagy; Metabolism.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


