10-Fold Increase in Hydrogen Atom Transfer Reactivity for a Series of S = 1 FeIV═O Complexes Over the S = 2 [(TQA)FeIV═O]2+ Complex via Entropic Lowering of Reaction Barriers by Secondary Sphere Cycloalkyl Substitution
IF 14.4
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
Nonheme iron enzymes utilize S = 2 iron(IV)-oxo intermediates as oxidants in biological oxygenations. In contrast, corresponding synthetic nonheme FeIV═O complexes characterized to date favor the S = 1 ground state that generally shows much poorer oxidative reactivity than their S = 2 counterparts. However, one intriguing exception found by Nam a decade ago is the S = 1 [FeIV(O)(Me3NTB)]2+ complex (Me3NTB = [tris((N-methyl-benzimidazol-2-yl)methyl)amine], 1O) with a hydrogen atom transfer (HAT) reactivity that is 70% that of the S = 2 [FeIV(O)(TQA)]2+ complex (TQA = tris(2-quinolylmethyl)amine, 3O). In our efforts to further explore this direction, we have unexpectedly uncovered a family of new S = 1 complexes with HAT reaction rates beyond the currently reported limits in the tripodal ligand family, surpassing oxidation rates found for the S = 2 [FeIV(O)(TQA)]2+ complex by as much as an order of magnitude. This is achieved simply by replacing the secondary sphere methyl groups of the Me3NTB ligand with larger cycloalkyl-CH2 (R groups in 2OR) moieties ranging from c-propylmethyl to c-hexylmethyl. These 2OR complexes show Mössbauer data at 4 K and 1H NMR spectra at 193 and 233 K that reveal S = 1 ground states, in line with DFT calculations. Nevertheless, they give rise to the most reactive synthetic nonheme oxoiron(IV) complexes found to date within the tripodal ligand family. Our DFT study indicates transition state stabilization through entropy effects, similar to enzymatic catalysis.
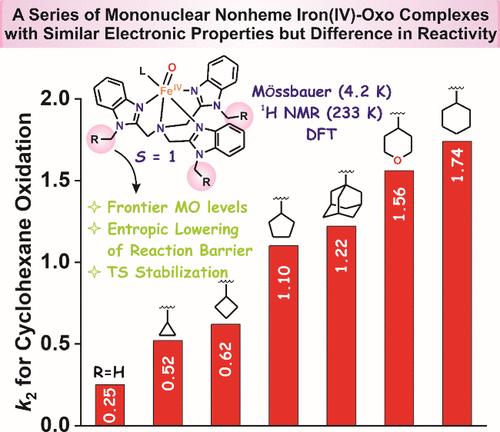
通过二级球环烷基取代对反应障碍的熵降作用,一系列 S = 1 FeIV═O 复合物的氢原子转移反应活性比 S = 2 [(TQA)FeIV═O]2+复合物提高 10 倍
非血红素铁酶利用S = 2铁(IV)-氧中间体作为生物氧合的氧化剂。相比之下,相应的合成非血红素FeIV = O络合物迄今表征的倾向于S = 1基态,通常显示出比S = 2对应物更差的氧化反应活性。然而,Nam在十年前发现的一个有趣的例外是S = 1 [FeIV(O)(Me3NTB)]2+配合物(Me3NTB = [tris((n -甲基-苯并咪唑-2-酰基)甲基)胺],其氢原子转移(HAT)反应活性是S = 2 [FeIV(O)(TQA)]2+配合物(TQA = tris(2-喹啉甲基)胺,30)的70%。在我们进一步探索这一方向的努力中,我们意外地发现了一个新的S = 1配合物家族,其HAT反应速率超过了目前报道的三足配体家族的极限,超过了S = 2 [FeIV(O)(TQA)]2+配合物的氧化速率多达一个数量级。这是通过用更大的环烷基- ch2 (2OR中的R基团)从c-丙基甲基到c-己基甲基取代Me3NTB配体的二级球甲基来实现的。这些2OR配合物显示了Mössbauer在4 K和193和233 K的1H NMR光谱数据,显示S = 1基态,与DFT计算一致。然而,它们产生了迄今为止在三足配体家族中发现的最具活性的合成非血红素氧铁(IV)复合物。我们的DFT研究表明,过渡态稳定通过熵效应,类似于酶催化。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
24.40
自引率
6.00%
发文量
2398
审稿时长
1.6 months
期刊介绍:
The flagship journal of the American Chemical Society, known as the Journal of the American Chemical Society (JACS), has been a prestigious publication since its establishment in 1879. It holds a preeminent position in the field of chemistry and related interdisciplinary sciences. JACS is committed to disseminating cutting-edge research papers, covering a wide range of topics, and encompasses approximately 19,000 pages of Articles, Communications, and Perspectives annually. With a weekly publication frequency, JACS plays a vital role in advancing the field of chemistry by providing essential research.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


