Aerobic Ammoxidation of Cyclic Ketones to Dinitrile Products with Copper-Based Catalysts
IF 14.4
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
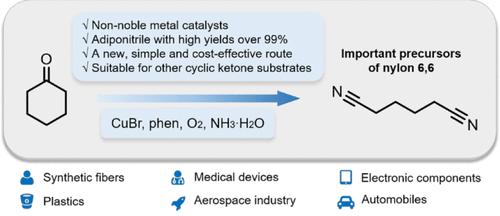
Adiponitrile (ADN) has wide applications, especially in the polymer industry. With the substantial and increasing global demand for ADN, effective production of ADN using safe and abundant starting materials is highly desirable but very challenging. Herein, we discovered that CuBr, combined with 1,10-phenanthroline (phen), could effectively promote the ammoxidation reaction of cyclohexanone to ADN with a yield of >99% using aqueous ammonia as the nitrogen source and O2 as the terminal oxidant under mild reaction conditions (80 °C, 5 atm O2). Moreover, cyclic ketones with various carbon numbers and substituent groups could also be converted into the corresponding dinitrile products with high yields. A detailed mechanistic study revealed that the reaction proceeded through a radical-mediated pathway, and the reason for the high selectivity to ADN was discussed. This study offers a new, simple, and cost-effective route to produce ADN and other dinitrile products.
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
24.40
自引率
6.00%
发文量
2398
审稿时长
1.6 months
期刊介绍:
The flagship journal of the American Chemical Society, known as the Journal of the American Chemical Society (JACS), has been a prestigious publication since its establishment in 1879. It holds a preeminent position in the field of chemistry and related interdisciplinary sciences. JACS is committed to disseminating cutting-edge research papers, covering a wide range of topics, and encompasses approximately 19,000 pages of Articles, Communications, and Perspectives annually. With a weekly publication frequency, JACS plays a vital role in advancing the field of chemistry by providing essential research.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


