Doublet Spin State Mediated Photoluminescence Upconversion in Organic Radical Donor-Triplet Acceptor Dyads
IF 14.4
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
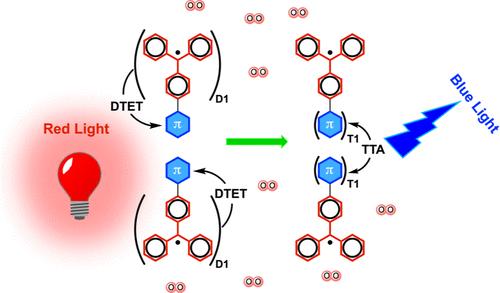
Donor–acceptor dyads are promising materials for improving triplet-sensitized photon upconversion due to faster intramolecular energy transfer (ET), which unfortunately competes with charge transfer (CT) dynamics. To circumvent the issue associated with CT, we propose a novel purely organic donor–acceptor dyad, where the CT character is confined within the donor moiety. In this work, we report the synthesis and characterization of a stable organic radical donor-triplet acceptor dyad (TTM–Cz–Per) consisting of the acceptor perylene (Per) linked to the donor (4-N-carbazolyl-2,6-dichlorophenyl)-bis(2,4,6-trichlorophenyl)methyl radical (TTM–Cz). Upon red-excitation of TTM–Cz–Per, the doublet emission of the donor (TTM–Cz) is significantly quenched, and a recorded delayed emission centered at ca. 490 nm was attributed to the fluorescence emission from the Per acceptor. Time-resolved transient absorption spectroscopy suggests doublet-to-triplet energy transfer (DTET) dynamics from the donor to the acceptor as the time constant τ for the donor transient species decreases from 21.47 ns for the TTM–Cz sensitizer to 8.73 ns for TTM–Cz–Per dyad. This process is accompanied by the appearance of a long-lived component with τ = 97.06 ns, which we ascribe to the triplet transient of the acceptor Per. Furthermore, computational results indicate that the DTET is intramolecular as computed spin densities of the quartet state show unpaired electrons of ρ ≈ 1 on the TTM-Cz donor and of ρ ≈ 2 on the acceptor Per. The present study highlights the possibility to employ doublet chromophoric systems for light-harvesting and energy upconversion, which can be further tailored for several optoelectronic applications.
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
24.40
自引率
6.00%
发文量
2398
审稿时长
1.6 months
期刊介绍:
The flagship journal of the American Chemical Society, known as the Journal of the American Chemical Society (JACS), has been a prestigious publication since its establishment in 1879. It holds a preeminent position in the field of chemistry and related interdisciplinary sciences. JACS is committed to disseminating cutting-edge research papers, covering a wide range of topics, and encompasses approximately 19,000 pages of Articles, Communications, and Perspectives annually. With a weekly publication frequency, JACS plays a vital role in advancing the field of chemistry by providing essential research.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


