Programming the Kinetics of Chemical Communication: Induced Fit vs Conformational Selection
IF 14.4
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
Life on Earth depends on chemical communication and the ability of biomolecular switches to integrate various chemical signals that trigger their activation or deactivation over time scales ranging from microseconds to days. The ability to similarly program and control the kinetics of artificial switches would greatly assist the design and optimization of future chemical and nanotechnological systems. Two distinct structure-switching mechanisms are typically employed by biomolecular switches: induced fit (IF) and conformational selection (CS). Despite 60 years of experimental and theoretical investigations, the kinetic and evolutive advantages of these two mechanisms remain unclear. Here, we have created a simple modular DNA switch that can operate through both mechanisms and be easily tuned and adapted to characterize its thermodynamic and kinetic parameters. We show that the fastest activation rate of a switch occurs when the ligand is able to bind its inactive conformation (IF). In contrast, we show that when the ligand can only bind the active conformation of the switch (CS), its activation rate can be easily programmed over many orders of magnitude by a simple tuning of its conformational equilibrium. We demonstrate the programming ability of both these mechanisms by designing a drug delivery vessel that can be programmed to release a drug over different time scales (>1000-fold). Overall, these findings provide a programmable strategy to optimize the kinetics of molecular systems and nanomachines while also illustrating how evolution may have taken advantage of IF and CS mechanisms to optimize the kinetics of biomolecular switches.
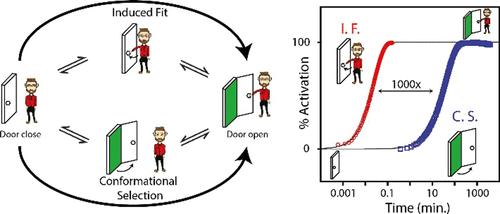
编程化学通讯动力学:诱导拟合与构象选择
地球上的生命依赖于化学通讯和生物分子开关整合各种化学信号的能力,这些化学信号会在从微秒到几天的时间尺度上触发它们的激活或灭活。类似地编程和控制人工开关动力学的能力将极大地有助于未来化学和纳米技术系统的设计和优化。生物分子开关通常采用两种不同的结构开关机制:诱导拟合(IF)和构象选择(CS)。尽管经过了60年的实验和理论研究,这两种机制的动力学和进化优势仍不清楚。在这里,我们创造了一个简单的模块化DNA开关,可以通过这两种机制操作,并且很容易调整和适应,以表征其热力学和动力学参数。我们表明,当配体能够结合其非活性构象(IF)时,开关的最快激活速率发生。相反,我们表明,当配体只能结合开关的活性构象(CS)时,通过简单调整其构象平衡,其激活率可以很容易地编程超过许多数量级。我们通过设计一种药物输送管道来证明这两种机制的编程能力,该管道可以在不同的时间尺度(1000倍)上编程释放药物。总的来说,这些发现提供了一种可编程的策略来优化分子系统和纳米机器的动力学,同时也说明了进化如何利用中频和CS机制来优化生物分子开关的动力学。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
24.40
自引率
6.00%
发文量
2398
审稿时长
1.6 months
期刊介绍:
The flagship journal of the American Chemical Society, known as the Journal of the American Chemical Society (JACS), has been a prestigious publication since its establishment in 1879. It holds a preeminent position in the field of chemistry and related interdisciplinary sciences. JACS is committed to disseminating cutting-edge research papers, covering a wide range of topics, and encompasses approximately 19,000 pages of Articles, Communications, and Perspectives annually. With a weekly publication frequency, JACS plays a vital role in advancing the field of chemistry by providing essential research.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


