Enantioselective α-C(sp3)–H Borylation of Masked Primary Alcohols Enabled by Iridium Catalysis
IF 15.6
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
Functional group-directed site- and enantioselective C(sp3)–H functionalization of alcohols or masked alcohols represents a formidable challenge. We herein report the first example of iridium-catalyzed asymmetric α-C(sp3)–H borylation of primary alcohol-derived carbamates by the judicious choice of directing groups. A variety of chiral borylated carbamates were obtained with good to high enantioselectivities. We also demonstrated the synthetic utility by taking advantage of the highly transformable feature of C–B bonds and the leaving ability of carbamates.
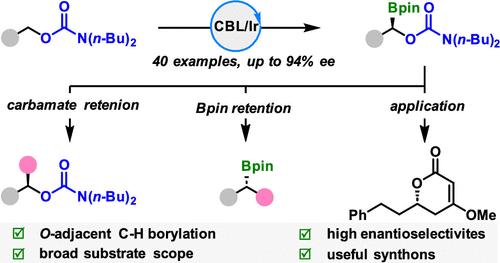
铱催化掩膜伯醇对映选择性α-C(sp3) -H硼化反应
官能团导向的位点和对映体选择性C(sp3) - h功能化醇或掩膜醇是一个艰巨的挑战。本文报道了铱催化的不对称α-C(sp3) -H氨基甲酸酯的定向基选择的第一个例子。得到了多种手性硼化氨基甲酸酯,具有良好的对映选择性。我们还通过利用C-B键的高度可转化特性和氨基甲酸酯的离开能力证明了合成的实用性。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
24.40
自引率
6.00%
发文量
2398
审稿时长
1.6 months
期刊介绍:
The flagship journal of the American Chemical Society, known as the Journal of the American Chemical Society (JACS), has been a prestigious publication since its establishment in 1879. It holds a preeminent position in the field of chemistry and related interdisciplinary sciences. JACS is committed to disseminating cutting-edge research papers, covering a wide range of topics, and encompasses approximately 19,000 pages of Articles, Communications, and Perspectives annually. With a weekly publication frequency, JACS plays a vital role in advancing the field of chemistry by providing essential research.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


