1,3-Dipolar Cycloaddition of SF5-Alkynes with Nonstabilized Diazo: Synthesis of Highly Substituted SF5-3H-Pyrazoles
IF 5
1区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
Abstract
This work presents the 1,3-dipolar cycloaddition of SF5-alkynes with nonstabilized diazo compounds under mild conditions, producing highly substituted SF5-3H-pyrazoles. Eighteen examples are given, with yields of up to 91%. The two regioisomers were obtained in ratios ranging from 27:73 to 73:27. The products can undergo a Van Alphen–Huttel rearrangement. DFT calculations were performed to understand the selectivities and rearrangements.
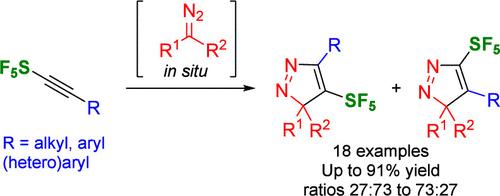
sf5 -炔与非稳定重氮的1,3-偶极环加成:高取代sf5 - 3h -吡唑的合成
本文研究了在温和条件下sf5 -炔与非稳定重氮化合物的1,3-偶极环加成反应,生成了高取代的sf5 - 3h -吡唑。给出了18个实例,产率高达91%。这两个区域异构体的比例在27:73到73:27之间。产物可以经历范阿尔芬-哈特尔重排。进行了DFT计算,以了解选择性和重排。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Organic Letters
化学-有机化学
CiteScore
9.30
自引率
11.50%
发文量
1607
审稿时长
1.5 months
期刊介绍:
Organic Letters invites original reports of fundamental research in all branches of the theory and practice of organic, physical organic, organometallic,medicinal, and bioorganic chemistry. Organic Letters provides rapid disclosure of the key elements of significant studies that are of interest to a large portion of the organic community. In selecting manuscripts for publication, the Editors place emphasis on the originality, quality and wide interest of the work. Authors should provide enough background information to place the new disclosure in context and to justify the rapid publication format. Back-to-back Letters will be considered. Full details should be reserved for an Article, which should appear in due course.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


