An Untethered and Formal Intermolecular Hexadehydro-Diels–Alder Reaction: Alkynylboronates with 2-(1,3-Butadiynyl)pyridines
IF 15.6
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
Journal of the American Chemical Society
Pub Date : 2024-12-06
DOI:10.1021/jacs.4c1162210.1021/jacs.4c11622
引用次数: 0
Abstract
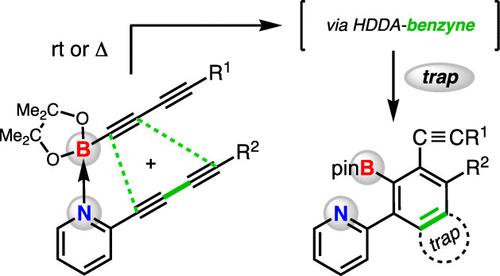
We show that 2-diynylpyridine and a Bpin-terminated monoyne or diyne will cross-react to form benzyne intermediates. These reactive intermediates are captured by various in situ trapping agents to give products of three-component reactions. Various control reactions, substrate modification, binding studies, and DFT analysis suggest that a small amount of a noncovalent Lewis acid–base complex is the active species within which the diyne and diynophile engage to produce the benzyne. Only a single isomeric benzyne is formed when a Bpin-diyne is used; this selectivity is rationalized by the geometric distortion seen in the DFT-computed diradical intermediate.
一种无系和正式的分子间六氢-Diels-Alder 反应:炔硼酸酯与 2-(1,3-丁二烯基)吡啶的反应
我们的研究表明,2-二炔基吡啶和以 Bpin 结尾的单炔或二炔会发生交叉反应,形成苄炔中间体。这些反应中间体会被各种原位捕获剂捕获,生成三组分反应的产物。各种控制反应、底物修饰、结合研究和 DFT 分析表明,少量非共价的路易斯酸碱复合物是活性物质,二炔和亲二炔在其中结合生成苄炔。当使用 Bpin 二炔时,只能形成单一异构体的苄烯;这种选择性可以通过 DFT 计算出的二叉中间体的几何畸变得到合理解释。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
24.40
自引率
6.00%
发文量
2398
审稿时长
1.6 months
期刊介绍:
The flagship journal of the American Chemical Society, known as the Journal of the American Chemical Society (JACS), has been a prestigious publication since its establishment in 1879. It holds a preeminent position in the field of chemistry and related interdisciplinary sciences. JACS is committed to disseminating cutting-edge research papers, covering a wide range of topics, and encompasses approximately 19,000 pages of Articles, Communications, and Perspectives annually. With a weekly publication frequency, JACS plays a vital role in advancing the field of chemistry by providing essential research.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


