Base-Free Borylation of Aryl Halides Enabled by Zn-Promoted Halide Abstraction
IF 5
1区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
Abstract
Herein, we report the palladium-catalyzed borylation of aryl halides (iodides or bromides) under base-free conditions utilizing a commercially available Lewis acidic mediator, Zn(OTf)2. Under these conditions, an array of electronically and functional-group-diverse aryl iodides and bromides undergo borylation to afford the corresponding aryl boronic esters in ≤82% isolated yields. Mechanistic investigations are consistent with Zn(OTf)2 enabling transmetalation between a cationic Pd(II)–Ar intermediate and B2pin2 via halide abstraction. Furthermore, stabilization of the cationic [ArPdII]+ complex with added [BArF4]− significantly improves the reaction efficiency with electron-poor arenes.
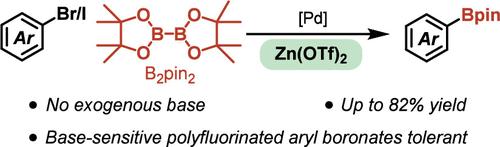
用锌促进卤化物萃取实现芳基卤化物的无碱硼化
本文报道了在无碱条件下,钯催化芳基卤化物(碘化物或溴化物)的硼化反应,利用市售的刘易斯酸性介质Zn(OTf)2。在这些条件下,一系列电子和官能团多样化的芳基碘化物和溴化物发生硼化反应,得到相应的芳基硼酯,分离产率≤82%。机理研究表明,Zn(OTf)2可以通过卤化物萃取在阳离子Pd(II) -Ar中间体和B2pin2之间发生金属转化。此外,加入[BArF4]−的阳离子[ArPdII]+配合物的稳定性显著提高了与缺电子芳烃的反应效率。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Organic Letters
化学-有机化学
CiteScore
9.30
自引率
11.50%
发文量
1607
审稿时长
1.5 months
期刊介绍:
Organic Letters invites original reports of fundamental research in all branches of the theory and practice of organic, physical organic, organometallic,medicinal, and bioorganic chemistry. Organic Letters provides rapid disclosure of the key elements of significant studies that are of interest to a large portion of the organic community. In selecting manuscripts for publication, the Editors place emphasis on the originality, quality and wide interest of the work. Authors should provide enough background information to place the new disclosure in context and to justify the rapid publication format. Back-to-back Letters will be considered. Full details should be reserved for an Article, which should appear in due course.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


