FON: An Innovative Fluorinated Group via Hydroetherification-Type Reactivity
IF 4.9
1区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
Abstract
An efficient strategy for preparing the novel O-difluoroalkylhydroxylamine fluorinated functional group, coined FON, is reported. This analogue of medicinally important β-phenethyl ether scaffolds in uniting gem-difluoro and N–O moieties is synthesized in one step via chemo- and regioselectivity metal-free hydroetherification-type additions. As shown, this unique mode of reactivity is realized for a diverse substrate scope and applied to gram-scale synthesis and site-selective deuterium incorporation. Lastly, a mechanistic understanding with implications in Brønsted acid catalysis is offered.
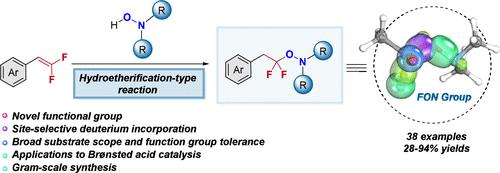
FON:通过氢醚化反应生成的创新型含氟基团
报告了一种制备新型 O-二氟烷基羟胺氟化官能团(又称 FON)的高效策略。通过化学和区域选择性无金属氢醚化型加成法,一步合成了这种具有重要药用价值的 β-苯乙基醚支架类似物,其结合了 gem-difluoro 和 N-O 两个分子。如图所示,这种独特的反应模式可用于多种底物范围,并可应用于克级合成和位点选择性氘结合。最后,还提供了对布伦司特酸催化的机理理解。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Organic Letters
化学-有机化学
CiteScore
9.30
自引率
11.50%
发文量
1607
审稿时长
1.5 months
期刊介绍:
Organic Letters invites original reports of fundamental research in all branches of the theory and practice of organic, physical organic, organometallic,medicinal, and bioorganic chemistry. Organic Letters provides rapid disclosure of the key elements of significant studies that are of interest to a large portion of the organic community. In selecting manuscripts for publication, the Editors place emphasis on the originality, quality and wide interest of the work. Authors should provide enough background information to place the new disclosure in context and to justify the rapid publication format. Back-to-back Letters will be considered. Full details should be reserved for an Article, which should appear in due course.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


