Late-Stage Tailoring Steps in the Biosynthesis of β-Rubromycin Involve Successive Terminal Oxidations, a Selective Hydroxyl Reduction, and Distinctive O-Methylations
IF 4.9
1区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
Abstract
β-Rubromycin (1) has a unique O-methylated naphthoquinone moiety and is an efficient inhibitor of human telomerase. Through in vivo and in vitro investigations, we elucidated the biosynthetic tailoring steps of compound 1, which involve the carboxyl terminal via successive oxidizations by RubU and RubO1/RubO2, O-methylation of the carboxyl terminal by RubX, and reduction of C-3′ hydroxyl by RubK. The final tautomerization of the naphthoquinone moiety is mediated by RubO, which anchors the transitional intermediate through O-methylation to form the naphthoquinone tautomer. The structure–activity relationship further revealed the significant influences of the terminal modification status on anticancer activities of rubromycins.
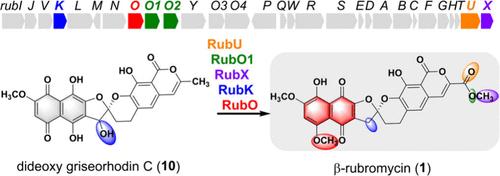
β-红霉素(1)具有独特的 O-甲基化萘醌分子,是一种高效的人类端粒酶抑制剂。通过体内和体外研究,我们阐明了化合物 1 的生物合成裁剪步骤,其中包括通过 RubU 和 RubO1/RubO2 相继氧化羧基末端,RubX 对羧基末端进行 O-甲基化,以及 RubK 对 C-3′ 羟基进行还原。萘醌分子的最终同分异构是由 RubO 介导的,RubO 通过 O-甲基化固定过渡中间体,形成萘醌同分异构体。结构-活性关系进一步揭示了末端修饰状态对红霉素类抗癌活性的重要影响。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Organic Letters
化学-有机化学
CiteScore
9.30
自引率
11.50%
发文量
1607
审稿时长
1.5 months
期刊介绍:
Organic Letters invites original reports of fundamental research in all branches of the theory and practice of organic, physical organic, organometallic,medicinal, and bioorganic chemistry. Organic Letters provides rapid disclosure of the key elements of significant studies that are of interest to a large portion of the organic community. In selecting manuscripts for publication, the Editors place emphasis on the originality, quality and wide interest of the work. Authors should provide enough background information to place the new disclosure in context and to justify the rapid publication format. Back-to-back Letters will be considered. Full details should be reserved for an Article, which should appear in due course.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


