Catalytic Asymmetric Oxidative Coupling between C(sp3)–H Bonds and Carboxylic Acids
IF 15.6
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
The direct enantioselective functionalization of C(sp3)–H bonds in organic molecules could fundamentally transform the synthesis of chiral molecules. In particular, the enantioselective oxidation of these bonds would dramatically change the production methods of chiral alcohols and esters, which are prevalent in natural products, pharmaceuticals, and fine chemicals. Remarkable advances have been made in the enantioselective construction of carbon–carbon and carbon–nitrogen bonds through the C(sp3)–H bond functionalization. However, the direct enantioselective formation of carbon–oxygen bonds from C(sp3)–H bonds remains a considerable challenge. We herein report a highly enantioselective C(sp3)–H bond oxidative coupling with carboxylic acids. The method applies to allylic and propargylic C–H bonds and employs various carboxylic acids as oxygenating agents. The method successfully synthesized a range of chiral esters directly from readily available alkenes and alkynes, greatly simplifying the synthesis of chiral esters and related alcohols.
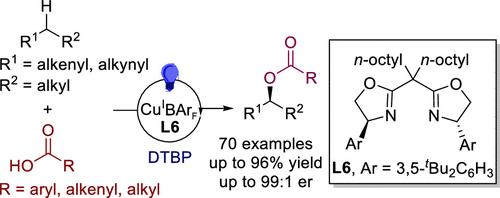
C(sp3) -H键与羧酸的催化不对称氧化偶联
有机分子中C(sp3) -H键的直接对映选择性功能化可以从根本上改变手性分子的合成。特别是,这些键的对映选择性氧化将极大地改变手性醇和酯的生产方法,这在天然产品,药品和精细化学品中普遍存在。C(sp3) -氢键功能化在碳碳键和碳氮键的对映选择性构建方面取得了显著进展。然而,从C(sp3) -H键直接对映选择性形成碳氧键仍然是一个相当大的挑战。我们在此报道了一个高度对映选择性的C(sp3) -H键与羧酸的氧化偶联。该方法适用于烯丙基和丙基C-H键,并采用各种羧酸作为氧合剂。该方法成功地从易得的烯烃和炔直接合成了一系列手性酯,大大简化了手性酯和相关醇的合成。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
24.40
自引率
6.00%
发文量
2398
审稿时长
1.6 months
期刊介绍:
The flagship journal of the American Chemical Society, known as the Journal of the American Chemical Society (JACS), has been a prestigious publication since its establishment in 1879. It holds a preeminent position in the field of chemistry and related interdisciplinary sciences. JACS is committed to disseminating cutting-edge research papers, covering a wide range of topics, and encompasses approximately 19,000 pages of Articles, Communications, and Perspectives annually. With a weekly publication frequency, JACS plays a vital role in advancing the field of chemistry by providing essential research.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


