Exploiting 19F NMR in a Multiplexed Assay for Small GTPase Activity
IF 15.6
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
Small GTPases (smG) are a 150-member family of proteins, comprising five subfamilies: Ras, Rho, Arf, Rab, and Ran-GTPases. These proteins function as molecular switches, toggling between two distinct nucleotide-bound states. Using traditional multidimensional heteronuclear NMR, even for single smGs, numerous experiments, high protein concentrations, expensive isotope labeling, and long analysis times are necessary. 19F NMR of fluorinated proteins or ligands can overcome these drawbacks. Using indole position-specific 19F labeling of the proteins, the activities of several smGs were measured in a multiplexed fashion. We investigated 4-, 5-, 6-, and 7-fluoro tryptophan containing smGs to study nucleotide binding. Distinct resonances for GDP- or GTP-bound states of three different 19F-labeled smGs, RhoA, K-Ras, and Rac1, were observed, and the kinetics of exchange and hydrolysis were measured. This multiplexed system will permit screening of nucleotide-specific ligands of smGs under true physiological conditions.
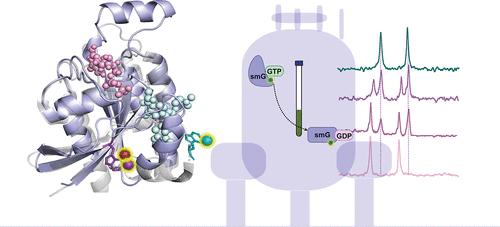
利用19F核磁共振多重分析小GTPase活性
小GTPases (Small GTPases, smG)是一个150个成员的蛋白家族,包括5个亚家族:Ras、Rho、Arf、Rab和Ran-GTPases。这些蛋白质起着分子开关的作用,在两种不同的核苷酸结合状态之间切换。使用传统的多维异核磁共振,即使是单个smGs,也需要大量的实验,高蛋白质浓度,昂贵的同位素标记和长时间的分析时间。氟化蛋白质或配体的19F核磁共振可以克服这些缺点。利用吲哚位置特异性19F标记蛋白质,以多重方式测量了几种smGs的活性。我们研究了含有smGs的4-、5-、6-和7-氟色氨酸来研究核苷酸结合。观察到三种不同19f标记的smGs (RhoA, K-Ras和Rac1)的GDP或gtp结合态的不同共振,并测量了交换和水解动力学。这种多路复用系统将允许在真实生理条件下筛选smGs的核苷酸特异性配体。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
24.40
自引率
6.00%
发文量
2398
审稿时长
1.6 months
期刊介绍:
The flagship journal of the American Chemical Society, known as the Journal of the American Chemical Society (JACS), has been a prestigious publication since its establishment in 1879. It holds a preeminent position in the field of chemistry and related interdisciplinary sciences. JACS is committed to disseminating cutting-edge research papers, covering a wide range of topics, and encompasses approximately 19,000 pages of Articles, Communications, and Perspectives annually. With a weekly publication frequency, JACS plays a vital role in advancing the field of chemistry by providing essential research.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


