Mechanism of C-3 Acyl Neighboring Group Participation in Mannuronic Acid Glycosyl Donors
IF 14.4
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
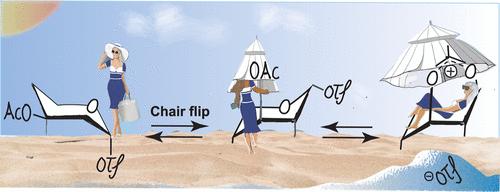
One of the main challenges in oligosaccharide synthesis is the stereoselective introduction of the glycosidic bond. In order to understand and control glycosylation reactions, thorough mechanistic studies are required. Reaction intermediates found by NMR spectroscopy often cannot explain the glycosylation’s stereochemical outcome. Hence, reactions may proceed through low-abundance reaction intermediates that are difficult to detect, according to a Curtin–Hammett scenario. We have previously observed that manno-type sugars can engage in C-3 acyl neighboring group participation. Herein, we report the detection of glycosyl dioxanium ions that result from C-3 neighboring group participation in mannuronic acid donors. Using a suite of exchange NMR techniques, we were able to dissect the kinetics of the conformational ring-flip that precedes C-3 acyl participation and the participation event itself in various manno-type sugars. Hence, this study provides a complete picture of mannosyl dioxanium ion formation.
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
24.40
自引率
6.00%
发文量
2398
审稿时长
1.6 months
期刊介绍:
The flagship journal of the American Chemical Society, known as the Journal of the American Chemical Society (JACS), has been a prestigious publication since its establishment in 1879. It holds a preeminent position in the field of chemistry and related interdisciplinary sciences. JACS is committed to disseminating cutting-edge research papers, covering a wide range of topics, and encompasses approximately 19,000 pages of Articles, Communications, and Perspectives annually. With a weekly publication frequency, JACS plays a vital role in advancing the field of chemistry by providing essential research.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


