Constructing Atomic Tungsten-Based Solid Frustrated-Lewis-Pair Sites with d-p Interactions for Selective CO2 Photoreduction
IF 14.4
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
Solid frustrated Lewis pair (FLP) shows remarkable advantages in the activation of small molecules such as CO2, owing to the strong orbital interactions between FLP sites and reactant molecules. However, most of the currently constructed FLP sites are randomly distributed and easily reunited on the surface of catalysts, resulting in a low utilization rate of FLP sites. Herein, atomic tungsten-based FLP (N···WSA FLP) sites are constructed for photocatalytic CO2 conversion through introducing W single-atoms into polymeric carbon nitride. In the atomically dispersed N···WSA FLP, the electron-deficient W single-atom acts as the Lewis acid (LA), and the adjacent electron-rich N atom acts as the Lewis base. Through the combination of various characterizations, including pyridine-IR, in situ diffuse reflectance infrared Fourier transform spectroscopy, CO2-temperature programmed desorption, and theoretical calculations, the positive effects of N···WSA FLP on photocatalytic CO2 reduction are well revealed. The N···WSA FLP can effectively adsorb CO2 to form an unusual W–O–C–N structure with significant d-p orbital interactions, which leads to an interesting “push–push” electron transfer effect. The π back-donation from W 5d to the antibonding orbital (2π) of CO2 realizes reverse electron transfer from the W single-atom to the O site, while the electrons are transferred from the electron-rich N site to the electropositive C site via Lewis acid–base interactions, therefore effectively breaking the C═O bond to activate CO2 molecules and boost CO2-to-CO performance. This work provides a brand new route for the research on high-efficiency activation of small molecules based on single-atom-based FLP catalysts.
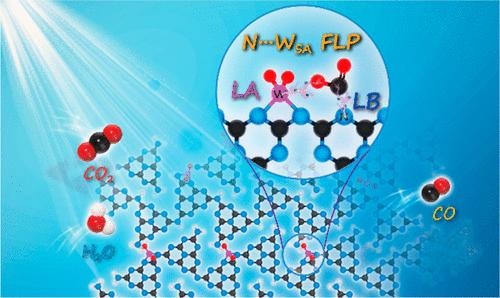
构建具有d-p相互作用的钨基固体受挫刘易斯对原子位用于选择性CO2光还原
固体受挫路易斯对(FLP)在活化二氧化碳等小分子方面具有显著优势,这是由于 FLP 位点与反应物分子之间具有很强的轨道相互作用。然而,目前构建的 FLP 位点大多随机分布,很容易在催化剂表面重聚,导致 FLP 位点的利用率较低。本文通过在聚合氮化碳中引入 W 单原子,构建了原子钨基 FLP(N--WSA FLP)位点,用于光催化二氧化碳转化。在原子分散的 N-WSA FLP 中,缺电子的 W 单原子充当路易斯酸(LA),而相邻的富电子 N 原子则充当路易斯碱。通过结合吡啶-红外光谱、原位漫反射红外傅立叶变换光谱、二氧化碳温控解吸和理论计算等多种表征方法,N--WSA FLP在光催化还原二氧化碳方面的积极作用得到了很好的揭示。N-WSA FLP能有效地吸附二氧化碳,形成不同寻常的W-O-C-N结构,并具有显著的d-p轨道相互作用,从而产生了有趣的 "推-推 "电子转移效应。从 W 5d 到 CO2 的反键轨道(2π)的π反向捐赠实现了从 W 单原子到 O 位点的反向电子转移,而电子则通过路易斯酸碱相互作用从电子丰富的 N 位点转移到电性正的 C 位点,从而有效地断开 C═O 键,激活 CO2 分子,提高 CO2 转化为 CO 的性能。这项工作为基于单原子的 FLP 催化剂高效活化小分子的研究提供了一条全新的途径。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
24.40
自引率
6.00%
发文量
2398
审稿时长
1.6 months
期刊介绍:
The flagship journal of the American Chemical Society, known as the Journal of the American Chemical Society (JACS), has been a prestigious publication since its establishment in 1879. It holds a preeminent position in the field of chemistry and related interdisciplinary sciences. JACS is committed to disseminating cutting-edge research papers, covering a wide range of topics, and encompasses approximately 19,000 pages of Articles, Communications, and Perspectives annually. With a weekly publication frequency, JACS plays a vital role in advancing the field of chemistry by providing essential research.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


