Bespoke Degradable Polymers: Modifying Cyclic Allyl Sulfide Lactones to Tune Polymer Degradation Rates and Lower Critical Solution Temperatures
IF 5.2
1区 化学
Q1 POLYMER SCIENCE
引用次数: 0
Abstract
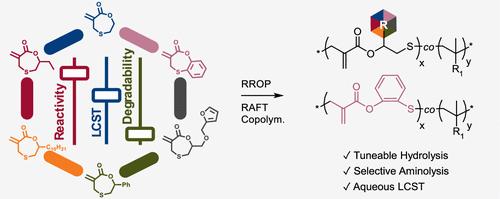
A method is presented to modify the degradation rates of copolymers made through vinyl- and radical ring-opening copolymerization. Six (including five novel) 7-membered cyclic allylic sulfide (CAS) lactones with varied functionality (H, ethyl, decyl, phenyl, furyl, and benzo) at the 2 (or 2/3) position were synthesized. Their radical copolymerization with hydroxypropyl methacrylamide (HPMAm), N-isopropylmethacrylamide, and oligo(ethylene glycol) methyl ether methacrylate (OEGMA) led to the inclusion of primary alkyl, secondary alkyl, benzyl, and phenyl esters into the backbone. The choice of CAS lactone comonomer allowed tuning the LCST-type cloud point of OEGMA copolymers, with substituents decreasing the cloud point in the order H < ethyl < decyl ≪ phenyl ∼ benzo. Hydrolytic degradation rate coefficients determined on HPMAm copolymers varied by a factor of 3.3, with better alcohol leaving groups leading to faster degradation. Backbone phenyl esters degraded through aminolysis, while benzyl and aliphatic esters were stable. Selective degradation was also achieved in block copolymers containing (oxo)esters in one block and thioesters (from thionolactone copolymerization) in the other. These findings demonstrate the tuning of physical properties and degradation behavior through the choice of comonomer enabling the design of degradable polymers with tailored properties.
定制可降解聚合物:修改环烯丙基硫内酯以调整聚合物降解率和降低临界溶液温度
本文介绍了一种改变乙烯基和自由基开环共聚物降解率的方法。我们合成了六种(包括五种新型)在 2(或 2/3)位具有不同官能团(H、乙基、癸基、苯基、呋喃基和苯基)的 7 元环烯丙基硫醚(CAS)内酯。它们与羟丙基甲基丙烯酰胺(HPMAm)、N-异丙基甲基丙烯酰胺和低聚(乙二醇)甲基醚甲基丙烯酸酯(OEGMA)进行自由基共聚,从而在骨架中加入了伯烷基、仲烷基、苄基和苯基酯。选择 CAS 内酯共聚物可以调整 OEGMA 共聚物的 LCST 型浊点,取代基按照 H < ethyl < decyl ≪ phenyl ≫ ≪ benzo 的顺序降低浊点。在 HPMAm 共聚物上测定的水解降解率系数相差 3.3 倍,醇离去基团越好,降解速度越快。骨架苯酯会通过氨解作用降解,而苄酯和脂肪族酯则比较稳定。在含有(氧化)酯的嵌段共聚物和含有硫代酯(由亚硫酰内酯共聚而成)的嵌段共聚物中也实现了选择性降解。这些研究结果表明,通过选择共聚单体可以调整物理特性和降解行为,从而设计出具有定制特性的可降解聚合物。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Macromolecules
工程技术-高分子科学
CiteScore
9.30
自引率
16.40%
发文量
942
审稿时长
2 months
期刊介绍:
Macromolecules publishes original, fundamental, and impactful research on all aspects of polymer science. Topics of interest include synthesis (e.g., controlled polymerizations, polymerization catalysis, post polymerization modification, new monomer structures and polymer architectures, and polymerization mechanisms/kinetics analysis); phase behavior, thermodynamics, dynamic, and ordering/disordering phenomena (e.g., self-assembly, gelation, crystallization, solution/melt/solid-state characteristics); structure and properties (e.g., mechanical and rheological properties, surface/interfacial characteristics, electronic and transport properties); new state of the art characterization (e.g., spectroscopy, scattering, microscopy, rheology), simulation (e.g., Monte Carlo, molecular dynamics, multi-scale/coarse-grained modeling), and theoretical methods. Renewable/sustainable polymers, polymer networks, responsive polymers, electro-, magneto- and opto-active macromolecules, inorganic polymers, charge-transporting polymers (ion-containing, semiconducting, and conducting), nanostructured polymers, and polymer composites are also of interest. Typical papers published in Macromolecules showcase important and innovative concepts, experimental methods/observations, and theoretical/computational approaches that demonstrate a fundamental advance in the understanding of polymers.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


