The Synthesis of Sulfonyl Fluoride Functionalized 2-Aminothiazoles Using a Diversity Oriented Clicking Strategy
IF 4.9
1区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
Abstract
We present a Diversity Oriented Clicking approach to synthesize a library of novel clickable N-substituted 2-aminothiazoles which serve as versatile hubs for SuFEx click chemistry diversification. Leveraging the spring-loaded reactivity of the 2-Substituted-Alkynyl-1-Sulfonyl Fluoride (SASF) connectors, the transformation is simple to perform, tolerant of a wide range of functionality, and regioselective for a single product. Finally, we propose a detailed stepwise reaction mechanism that is supported by experimental and computational analysis.
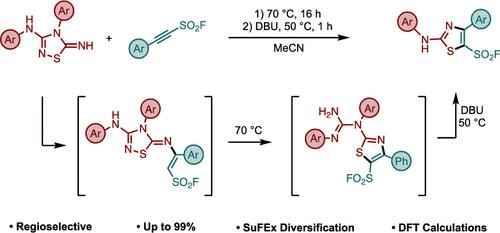
我们提出了一种以多样性为导向的点击方法,用于合成新型可点击的 N-取代 2-氨基噻唑库,这些噻唑可作为 SuFEx 点击化学多样化的多功能枢纽。利用 2-取代-炔基-1-磺酰氟 (SASF) 连接器的弹簧反应性,该转化过程简单易行,可容忍广泛的官能度,并对单一产物具有区域选择性。最后,我们提出了一个详细的分步反应机制,并得到了实验和计算分析的支持。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Organic Letters
化学-有机化学
CiteScore
9.30
自引率
11.50%
发文量
1607
审稿时长
1.5 months
期刊介绍:
Organic Letters invites original reports of fundamental research in all branches of the theory and practice of organic, physical organic, organometallic,medicinal, and bioorganic chemistry. Organic Letters provides rapid disclosure of the key elements of significant studies that are of interest to a large portion of the organic community. In selecting manuscripts for publication, the Editors place emphasis on the originality, quality and wide interest of the work. Authors should provide enough background information to place the new disclosure in context and to justify the rapid publication format. Back-to-back Letters will be considered. Full details should be reserved for an Article, which should appear in due course.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


