Mechanistic Insights into Glycerol Ketalization to Glycerol Levulinate Ketal over USY Molecular Sieve
IF 3.3
3区 化学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
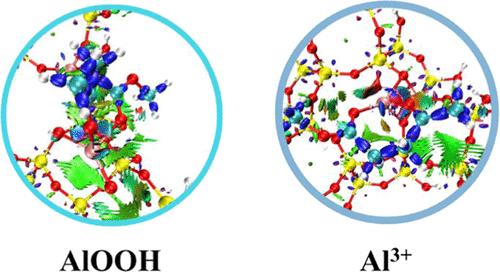
Efficient ketalization of glycerol and alkyl levulinate to high-value-added glycerol levulinate ketal (GLK) and insights into the catalytic mechanism are of great significance but remain challenging without a clear understanding of active sites. Herein, the ketalization of glycerol with methyl levulinate (ML) and ethyl levulinate (EL) to methyl glycerol levulinate ketal (MLK) and ethyl glycerol levulinate ketal (ELK) over USY molecular sieves was reported. Under the optimal conditions, the yields of MLK and ELK can reach 96%. A kinetic study indicated that the ketalization of alkyl levulinate and glycerol to GLK followed the pseudo-second-order kinetic model, and the activation energy for MLK synthesis (154.87 kJ·mol–1) over USY(6) was lower than that of ELK (168.03 kJ·mol–1) over USY(10). Density functional theory calculations revealed that AlOOH sites and Al3+ sites in USY exhibit identical catalytic activity during the synthesis of MLK (or ELK). Furthermore, the formation of an ether intermediate (IM2) from the acyl group of ML attacked by the GLY-terminated hydroxyl was the rate-determining step of MLK production on the AlOOH sites, while the intramolecular cyclization of an intermediate IM3 containing a carbocation to form ELK was the rate-determining step of EL ketalization on the Al3+ sites.
USY分子筛上甘油酮化制乙酰丙酸甘油酮的机理研究
高效地将甘油和乙酰丙酸烷基酯酮化为高附加值的甘油乙酰丙酸酮(GLK)并深入了解其催化机理具有重要意义,但在不清楚活性位点的情况下仍具有挑战性。本文报告了甘油与乙酰丙酸甲酯(ML)和乙酰丙酸乙酯(EL)在 USY 分子筛上发生酮化反应,生成甘油乙酰丙酸甲酯(MLK)和甘油乙酰丙酸乙酯(ELK)。在最佳条件下,MLK 和 ELK 的产率可达 96%。动力学研究表明,乙酰丙酸烷基酯和甘油酮化为 GLK 的过程遵循伪二阶动力学模型,在 USY(6) 上合成 MLK 的活化能(154.87 kJ-mol-1)低于在 USY(10) 上合成 ELK 的活化能(168.03 kJ-mol-1)。密度泛函理论计算显示,USY 中的 AlOOH 位点和 Al3+ 位点在合成 MLK(或 ELK)时表现出相同的催化活性。此外,在 AlOOH 位点上,ML 的酰基受到 GLY 端羟基的攻击而形成醚中间体(IM2)是 MLK 生成的决定性步骤,而在 Al3+ 位点上,含有碳位的中间体 IM3 分子内环化形成 ELK 是 EL 酮化的决定性步骤。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

The Journal of Physical Chemistry C
化学-材料科学:综合
CiteScore
6.50
自引率
8.10%
发文量
2047
审稿时长
1.8 months
期刊介绍:
The Journal of Physical Chemistry A/B/C is devoted to reporting new and original experimental and theoretical basic research of interest to physical chemists, biophysical chemists, and chemical physicists.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


