Crystal-Phase Engineering of PdCu Nanoparticles for Catalytic Reduction of NO by CO
IF 3.2
3区 化学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
Tuning the crystal phase of bimetallic nanoparticles has emerged as a promising strategy to boost their catalytic performance, but identifying the active site at the single-nanoparticle scale is rarely done and remains challenging. Here, the crystal phase of a PdCu single nanoparticle, spatially confined by a silica shell, was mediated between the ordered body-centered cubic (B2) phase and the disordered face-centered cubic (A1) phase. During the crystal-phase transition, the porous silica shell prevented the bimetallic nanoparticles from sintering under reactive gases and at elevated temperatures, enabling us to alter the crystal phase while keeping the particle size and atomic composition unchanged. Combined microscopic and spectroscopic characterizations revealed that the B2 particle was enclosed predominantly by the {110} facets over which Pd and Cu atoms were populated alternatively, while the A1 particle exposed mainly the {111} facets terminated by a random distribution of Pd and Cu atoms. When applied to catalyze NO reduction by CO, the B2 particle showed a much higher activity with a reaction rate of 4.5 times greater than that of the A1 particle. It was proposed that the orderly arranged Pd and Cu atoms on the {110} facets, exposed by the B2 particle, favored the coadsorption of NO and CO and further facilitated the dissociation of NO as the rate-determining step in the reaction network.
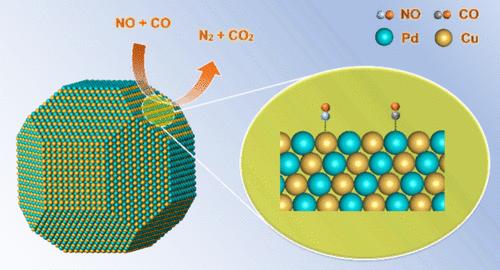
CO催化还原NO的PdCu纳米颗粒的晶相工程研究
调整双金属纳米粒子的晶相已成为提高其催化性能的一种有前途的策略,但在单纳米粒子尺度上确定活性位点的工作很少进行,而且仍然具有挑战性。在这里,钯铜单个纳米粒子的晶相被二氧化硅壳在空间上限制,介于有序的体心立方(B2)相和无序的面心立方(A1)相之间。在晶相转变过程中,多孔二氧化硅壳阻止了双金属纳米粒子在活性气体和高温下烧结,使我们能够在改变晶相的同时保持粒度和原子成分不变。显微镜和光谱综合表征显示,B2 颗粒主要由{110}面包围,钯原子和铜原子交替填充在{110}面上,而 A1 颗粒主要暴露在{111}面上,钯原子和铜原子随机分布。在催化一氧化碳还原一氧化氮时,B2 粒子显示出更高的活性,反应速率是 A1 粒子的 4.5 倍。据推测,B2 粒子露出的{110}面上有序排列的 Pd 和 Cu 原子有利于 NO 和 CO 的共吸附,并进一步促进了 NO 的解离,这是反应网络中决定速率的一步。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

The Journal of Physical Chemistry C
化学-材料科学:综合
CiteScore
6.50
自引率
8.10%
发文量
2047
审稿时长
1.8 months
期刊介绍:
The Journal of Physical Chemistry A/B/C is devoted to reporting new and original experimental and theoretical basic research of interest to physical chemists, biophysical chemists, and chemical physicists.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


