Contrasteric Glycosylations of Cotylenol and 1,2-Diols by Virtual Linker Selection
IF 14.4
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
Many terpene glycosides exhibit contrasteric patterns of 1,2-diol glycosylation in which the more hindered alcohol bears a sugar; protection of the less hindered alcohol only increases steric repulsion. Here, we report a method for contrasteric glycosylation using a new sugar-linker that forms a cleavable, 10-membered ring with high efficiency, leading to syntheses of cotylenin E, J, and ISIR-050. Linker selection was aided by DFT calculations of side reactions and stereoselectivity, as well as conformational analyses using autoDFT, a Python script that converts SMILES strings to DFT-optimized conformational ensembles.
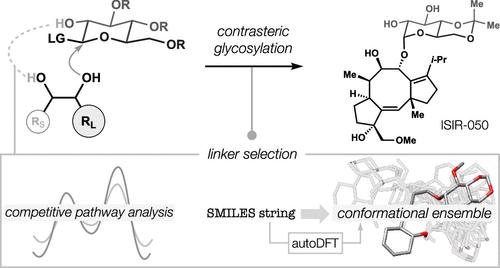
通过虚拟连接体选择实现儿茶酚和 1,2-二醇的对位糖基化
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
24.40
自引率
6.00%
发文量
2398
审稿时长
1.6 months
期刊介绍:
The flagship journal of the American Chemical Society, known as the Journal of the American Chemical Society (JACS), has been a prestigious publication since its establishment in 1879. It holds a preeminent position in the field of chemistry and related interdisciplinary sciences. JACS is committed to disseminating cutting-edge research papers, covering a wide range of topics, and encompasses approximately 19,000 pages of Articles, Communications, and Perspectives annually. With a weekly publication frequency, JACS plays a vital role in advancing the field of chemistry by providing essential research.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


