Ligand Triplet Energy Transfer from Perylene Diimide Derivatives to PbS Quantum Dots in Solution
IF 3.3
3区 化学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
A novel strategy has been devised to regulate the rate of triplet energy transfer by manipulating the energy level difference between the organic singlet fission donors and inorganic acceptors. The triplet energy donor PBI-OMe and acceptor PbS quantum dots are interconnected through carboxyl groups, resulting in varying degrees of orbital coupling between quantum dots of different energy levels and the donor. This is achieved through energy level diagrams and triplet energy transfer rates. The TET rate constants of PBI-OMe 0.91, PBI-OMe 1.29, and PBI-OMe 1.45 with different band gaps calculated are 2.63 × 109, 6.59 × 109, and 7.97 × 109 s–1 by time-resolved absorption spectroscopy, respectively. We confirmed that the TET rate from PBI-OMe to PbS QDs is limited by the degree of orbital coupling between PBI-OMe and PbS QDs, and that within a certain band gap range, samples with higher band gaps have better orbital coupling and achieve faster TET rates. Our work provides a straightforward and controllable platform to investigate the interfacial energy transfer process between SF materials and inorganic semiconductors, offering a new way to design molecule–nanocrystal combinations with high photovoltaic conversion efficiency and potential applications in other optoelectronic fields.
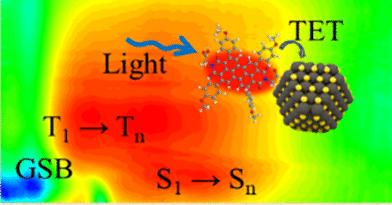
配体三重能从过二亚胺二衍生物向溶液中的 PbS 量子点转移
我们设计了一种新颖的策略,通过操纵有机单重裂变供体和无机受体之间的能级差来调节三重能转移率。三重能供体 PBI-OMe 和受体 PbS 量子点通过羧基相互连接,导致不同能级的量子点与供体之间产生不同程度的轨道耦合。这可以通过能级图和三重能转移率来实现。通过时间分辨吸收光谱,计算出不同带隙的 PBI-OMe 0.91、PBI-OMe 1.29 和 PBI-OMe 1.45 的 TET 速率常数分别为 2.63 × 109、6.59 × 109 和 7.97 × 109 s-1。我们证实,从 PBI-OMe 到 PbS QDs 的 TET 速率受限于 PBI-OMe 与 PbS QDs 之间的轨道耦合程度,在一定的带隙范围内,带隙越高的样品轨道耦合越好,TET 速率越快。我们的工作为研究 SF 材料与无机半导体之间的界面能量转移过程提供了一个直接、可控的平台,为设计具有高光电转换效率的分子-纳米晶体组合以及在其他光电领域的潜在应用提供了一条新途径。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

The Journal of Physical Chemistry C
化学-材料科学:综合
CiteScore
6.50
自引率
8.10%
发文量
2047
审稿时长
1.8 months
期刊介绍:
The Journal of Physical Chemistry A/B/C is devoted to reporting new and original experimental and theoretical basic research of interest to physical chemists, biophysical chemists, and chemical physicists.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


