CO2 Poisoning of CNx Catalysts for the Oxygen Reduction Reaction
IF 3.3
3区 化学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
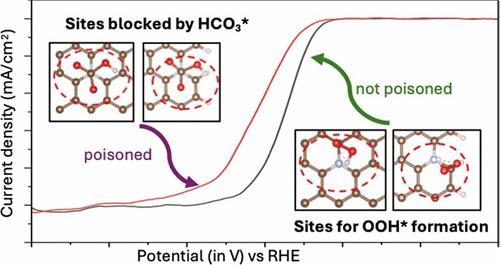
CNx catalysts show promising activity and stability for the oxygen reduction reaction (ORR) under acidic conditions, but the nature of the active site is still under debate. ORR on CNx has been found to be resistant to common poisons such as CO, H2S, and CN. In this study, we demonstrate that bubbling CO2 in the electrolyte can lead to the partial poisoning of CNx for ORR activity. Cyclic voltammetry (CV) experiments show a partial decrease in the ORR activity for CNx catalysts after bubbling CO2 through a 0.1 M HClO4 electrolyte. The relative stability of CO2-derived species (CO2*, H2CO3*, HCO3*, and CO3*) on 13 CNx site models at 1.0 V-RHE was examined using density functional theory (DFT). The calculations predict that HCO3 is favored adjacent to the N species on several CNx site models and CO3 is favored on pyrrolic sites. Difference spectra of the N 1s from near-ambient pressure X-ray photoelectron spectroscopy (NAP-XPS) of the ex-situ poisoned CNx and pristine CNx shows a shift in binding energies of N species that qualitatively match the DFT N 1s binding energy shifts due to HCO3/CO3 on the CNx site models. DFT predicts that these HCO3 surface species can block and delay the initial step of the ORR on several CNx sites.
氧还原反应中CNx催化剂的CO2中毒
CNx催化剂在酸性条件下表现出良好的氧还原反应(ORR)活性和稳定性,但活性位点的性质仍存在争议。CNx上的ORR已被发现能够抵抗常见的毒物,如CO、H2S和CN。在这项研究中,我们证明了电解液中冒泡的CO2可以导致CNx对ORR活性的部分中毒。循环伏安(CV)实验表明,在0.1 M HClO4电解液中鼓泡CO2后,CNx催化剂的ORR活性部分下降。利用密度泛函理论(DFT)研究了1.0 V-RHE条件下13个CNx位点模型上CO2衍生物质(CO2*、H2CO3*、HCO3*和CO3*)的相对稳定性。计算结果表明,在几种CNx位点模型中,HCO3倾向于靠近N种,而CO3倾向于靠近吡啶类位点。脱位毒化CNx和原始CNx的近环境压力x射线光电子能谱(NAP-XPS)差谱显示,n1s的结合能的变化与DFT n1s结合能的变化在CNx位点模型上由HCO3/CO3引起的变化有质的匹配。DFT预测这些HCO3表面物质可以阻断和延迟几个CNx位点上ORR的初始步骤。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

The Journal of Physical Chemistry C
化学-材料科学:综合
CiteScore
6.50
自引率
8.10%
发文量
2047
审稿时长
1.8 months
期刊介绍:
The Journal of Physical Chemistry A/B/C is devoted to reporting new and original experimental and theoretical basic research of interest to physical chemists, biophysical chemists, and chemical physicists.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


