IrO2/MnO2 metal oxide-support interaction enables robust acidic water oxidation
IF 9.7
1区 化学
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
The sluggish kinetics, poor stability, and high iridium loading in acidic oxygen evolution reaction (OER) present significant challenges for proton exchange membrane water electrolyzers (PEMWE). While supported catalysts can enhance the utilization and activity of Ir atoms, they often fail to mitigate the detrimental effects of over-oxidation and dissolution of Ir. Here, we leverage the redox properties of the Mn3+/Mn4+ couple as electronic modulators to develop a low-iridium, durable electrocatalyst for acidic OER. Specifically, IrO2 nanoparticles are anchored onto MnO2 nanowires (denoted as IrO2/MnO2), through a molten salt-assisted synthesis method. This optimized IrO2/MnO2 electrocatalyst features a substantially reduced iridium content and enhanced electronic structure due to strong metal-support interactions. Remarkably, the IrO2/MnO2 catalyst demonstrates 7-fold increase in intrinsic activity and superior durability compared to commercial IrO2. Both theoretical and experimental results indicate that dynamic electron transfer between Ir and Mn facilitates the rapid formation of highly oxidized iridium sites while simultaneously preventing excessive oxidation, thereby enhancing both the kinetics and stability for OER. A PEMWE utilizing IrO2/MnO2 as the anode catalyst achieves 2000 mA cm−2 @ 1.89 V without requiring supporting acidic electrolyte. Importantly, the PEMWE exhibits negligible degradation under harsh industrial operating conditions (1000 mA cm−2) with an Ir loading as low as 0.5 mg cm−2, while maintaining a low energy consumption of 45.58 kWh kg−1 H2, corresponding to the green hydrogen production cost of $0.9 kg−1 H2, significantly lower than the 2026 US-DOE target, underscoring its potential for practical application.
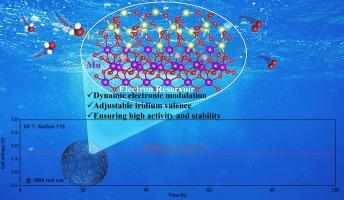
IrO2/MnO2 金属氧化物与支持物的相互作用实现了强酸性水氧化。
酸性析氧反应(OER)动力学慢、稳定性差、含铱量高,对质子交换膜水电解槽(PEMWE)提出了重大挑战。虽然负载型催化剂可以提高Ir原子的利用率和活性,但它们往往不能减轻Ir的过度氧化和溶解的有害影响。在这里,我们利用Mn3+/Mn4+对的氧化还原特性作为电子调制器来开发一种低铱、耐用的酸性OER电催化剂。具体来说,通过熔盐辅助合成方法,将IrO2纳米颗粒固定在MnO2纳米线上(表示为IrO2/MnO2)。这种优化后的IrO2/MnO2电催化剂具有显著降低铱含量和增强电子结构的特点,这是由于强金属支撑相互作用。值得注意的是,与商用IrO2相比,IrO2/MnO2催化剂的内在活性提高了7倍,耐久性也更好。理论和实验结果都表明,Ir和Mn之间的动态电子转移促进了高氧化铱位点的快速形成,同时防止了过度氧化,从而提高了OER的动力学和稳定性。使用IrO2/MnO2作为阳极催化剂的PEMWE在不需要辅助酸性电解质的情况下达到2000 mA cm-2 @ 1.89 V。重要的是,PEMWE在恶劣的工业操作条件下(1000 mA cm-2), Ir负载低至0.5 mg cm-2,表现出可以忽略不计的降解,同时保持45.58 kWh kg-1 H2的低能耗,对应于0.9 kg-1 H2的绿色制氢成本,显着低于2026年美国能源部的目标,强调了其实际应用潜力。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
16.10
自引率
7.10%
发文量
2568
审稿时长
2 months
期刊介绍:
The Journal of Colloid and Interface Science publishes original research findings on the fundamental principles of colloid and interface science, as well as innovative applications in various fields. The criteria for publication include impact, quality, novelty, and originality.
Emphasis:
The journal emphasizes fundamental scientific innovation within the following categories:
A.Colloidal Materials and Nanomaterials
B.Soft Colloidal and Self-Assembly Systems
C.Adsorption, Catalysis, and Electrochemistry
D.Interfacial Processes, Capillarity, and Wetting
E.Biomaterials and Nanomedicine
F.Energy Conversion and Storage, and Environmental Technologies

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


