Methionine-driven YTHDF1 expression facilitates bladder cancer progression by attenuating RIG-I-modulated immune responses and enhancing the eIF5B-PD-L1 axis
IF 15.4
1区 生物学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
Abstract
The impact of amino acids on tumor immunotherapy is gradually being uncovered. In this study, we screened various essential and non-essential amino acids and found that methionine enhances mRNA methylation and reduced the activation of Type I interferon pathway in bladder cancer. Through RNA sequencing, point mutations, MB49 mouse tumor models, and single-cell RNA sequencing, we demonstrated that high methionine levels elevate the expression of m6A reader YTHDF1, promoting the degradation of RIG-I, thereby inhibiting the RIG-I/MAVS-mediated IFN-I pathway and reducing the efficacy of tumor immunotherapy. Additionally, immunoprecipitation and mass spectrometry revealed that YTHDF1 binds to the eukaryotic translation initiation factor eIF5B, which acts on PD-L1 mRNA to enhance its translation and promote immune evasion. By intravesical administration of oncolytic bacteria VNP20009, we effectively depleted methionine locally, significantly prolonging mouse survival and enhancing immune cell infiltration and differentiation within tumors. Multiplex immunofluorescence assays in bladder cancer immunotherapy patients confirmed our findings. Our research elucidates two mechanisms by which methionine inhibits bladder cancer immunotherapy and proposes a targeted methionine depletion strategy that advances research while minimizing nutritional impact on patients.
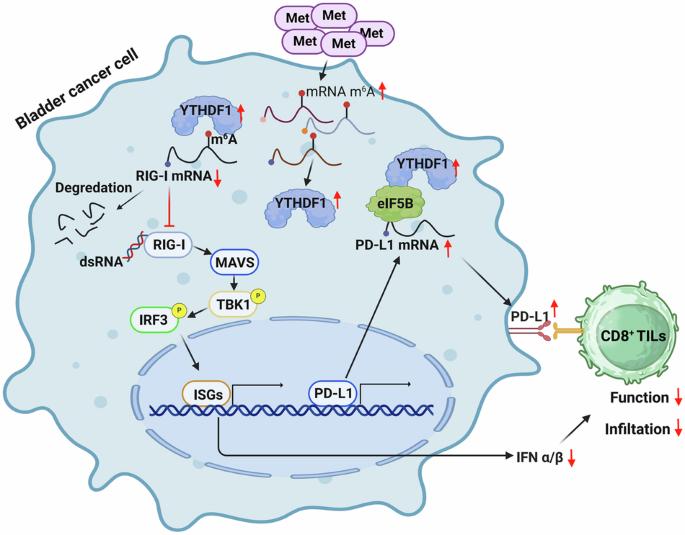
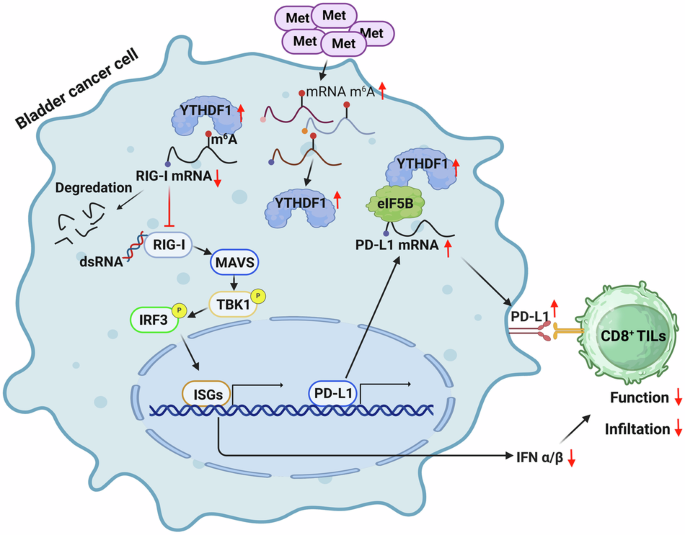
蛋氨酸驱动的YTHDF1表达通过减弱rig - i调节的免疫反应和增强eIF5B-PD-L1轴促进膀胱癌的进展
氨基酸对肿瘤免疫治疗的影响正逐渐被发现。在本研究中,我们筛选了多种必需和非必需氨基酸,发现蛋氨酸可以增强膀胱癌mRNA甲基化,降低I型干扰素通路的激活。通过RNA测序、点突变、MB49小鼠肿瘤模型和单细胞RNA测序,我们证明了高水平的甲硫氨酸可提高m6A读取器YTHDF1的表达,促进RIG-I的降解,从而抑制RIG-I/ mavs介导的IFN-I通路,降低肿瘤免疫治疗的疗效。此外,免疫沉淀和质谱分析显示,YTHDF1与真核翻译起始因子eIF5B结合,该因子作用于PD-L1 mRNA,增强其翻译并促进免疫逃避。通过体内给药溶瘤细菌VNP20009,我们有效地局部消耗了蛋氨酸,显著延长了小鼠的生存时间,增强了肿瘤内免疫细胞的浸润和分化。膀胱癌免疫治疗患者的多重免疫荧光试验证实了我们的发现。我们的研究阐明了蛋氨酸抑制膀胱癌免疫治疗的两种机制,并提出了一种有针对性的蛋氨酸消耗策略,在推进研究的同时最大限度地减少对患者的营养影响。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Cell Death and Differentiation
生物-生化与分子生物学
CiteScore
24.70
自引率
1.60%
发文量
181
审稿时长
3 months
期刊介绍:
Mission, vision and values of Cell Death & Differentiation:
To devote itself to scientific excellence in the field of cell biology, molecular biology, and biochemistry of cell death and disease.
To provide a unified forum for scientists and clinical researchers
It is committed to the rapid publication of high quality original papers relating to these subjects, together with topical, usually solicited, reviews, meeting reports, editorial correspondence and occasional commentaries on controversial and scientifically informative issues.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


