Comprehensive Experimental and Theoretical Study on the Adsorption and Corrosion Inhibition Efficiency of Pyronin B for Mild Steel Protection in HCl Solution
IF 3.2
3区 化学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
Mild steel (MS) is one of the most widely used materials in industry. But, this metal corrodes easily, especially in acidic solutions. Therefore, protection of this metal is critical. The use of inhibitors is one of the most practical and economical ways of achieving this. The corrosion inhibition performance of Pyronin B (PyB) for the protection of mild steel (MS) was investigated in 1 M HCl solution using the change of open circuit potential with exposure time (Eocp-t), potentiodynamic polarization, electrochemical impedance spectroscopy (EIS) and linear polarization resistance (LPR) techniques. The surface of the MS was examined by scanning electron microscopy (SEM), energy dispersive X-ray spectroscopy (EDX), atomic force microscopy (AFM) and contact angle measurements. The electrochemical stability of the PyB film formed on the MS surface was investigated by cyclic voltammetry (CV). The excess surface charge of the metal in the inhibited solution was estimated with the help of EIS studies. The experimental data were supported by theoretical approaches such as density functional theory (DFT) calculations, molecular dynamics (MD) and Monte Carlo simulations. It was found that PyB forms a uniformly distributed, dense, and protective organic film on the steel surface. The PyB molecules interact with the MS surface through a combination of physical and chemical interactions, the latter being dominant. The PyB acts as a mixed-type inhibitor, with a more pronounced effect on the anodic mechanism. Its corrosion inhibition efficiency depends on its concentration, reaching 95.9% efficiency at 0.01 mM. The inhibitor acts as an inhibitor without altering the mechanism of the cathodic reaction and by altering the mechanism of the anodic reaction. The overall corrosion reaction is kinetically controlled. The surface film exhibits very high electrochemical stability under potentiodynamic conditions. Theoretical approaches supported the experimental results and indicated superior inhibitory capability of PyB. The chemical reactivity of the studied inhibitor system was explained in the light of Conceptual Density Functional Theory based calculations and electronic structure principles. The binding energy, which reflects the power of the interaction between Fe(110) surface and PyB, was found to be 3.43 eV. Binding energies calculated by both DFT and MD and MC simulations support that the adsorption of the inhibitor is chemical, as noted in the experimental section.
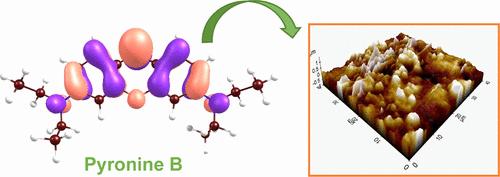
Pyronin B在HCl溶液中对低碳钢的吸附缓蚀效果的综合实验与理论研究
低碳钢(MS)是工业上应用最广泛的材料之一。但是,这种金属很容易被腐蚀,尤其是在酸性溶液中。因此,保护这种金属是至关重要的。使用抑制剂是实现这一目标的最实用和最经济的方法之一。采用开路电位随暴露时间的变化(Eocp-t)、动电位极化、电化学阻抗谱(EIS)和线性极化电阻(LPR)技术研究了Pyronin B (PyB)在1 M HCl溶液中的缓蚀保护性能。通过扫描电子显微镜(SEM)、能量色散x射线能谱(EDX)、原子力显微镜(AFM)和接触角测量对质谱表面进行了检测。采用循环伏安法(CV)研究了在质谱表面形成的PyB膜的电化学稳定性。在EIS研究的帮助下,估计了抑制溶液中金属的过量表面电荷。实验数据得到了密度泛函理论(DFT)计算、分子动力学(MD)和蒙特卡罗模拟等理论方法的支持。结果表明,PyB在钢表面形成一层分布均匀、致密、具有保护作用的有机薄膜。PyB分子通过物理和化学相互作用与质谱表面相互作用,后者占主导地位。PyB作为混合型缓蚀剂,对阳极机制的影响更为明显。其缓蚀效率取决于其浓度,在0.01 mM处达到95.9%的缓蚀效率。缓蚀剂在不改变阴极反应机理的情况下,通过改变阳极反应机理来起到缓蚀剂的作用。整个腐蚀反应是动力学控制的。该表面膜在动电位条件下表现出很高的电化学稳定性。理论方法支持实验结果,表明PyB具有良好的抑制能力。根据基于概念密度泛函理论的计算和电子结构原理,解释了所研究的缓蚀剂体系的化学反应性。反映Fe(110)表面与PyB相互作用强度的结合能为3.43 eV。DFT、MD和MC模拟计算的结合能支持抑制剂的化学吸附,如实验部分所述。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

The Journal of Physical Chemistry C
化学-材料科学:综合
CiteScore
6.50
自引率
8.10%
发文量
2047
审稿时长
1.8 months
期刊介绍:
The Journal of Physical Chemistry A/B/C is devoted to reporting new and original experimental and theoretical basic research of interest to physical chemists, biophysical chemists, and chemical physicists.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


