Gas-Phase Impurities in Hydrogen: DFT Calculations and Experimental Analysis of Interactions with Gadolinium Hydride Surfaces
IF 3.2
3区 化学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
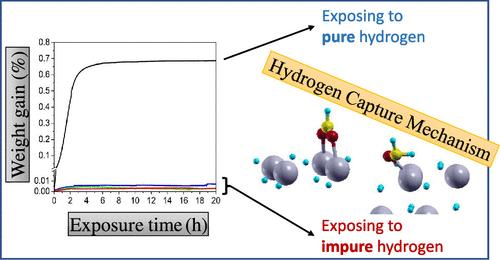
The interactions between gaseous hydrogen and metallic compounds are of significant basic scientific interest as well as important in various practical applications, including metal hydride formation, energy storage systems, and catalysis. Typically, the initial hydrogenation process involves surface hydrogen dissociation on the native oxide layers followed by atomic hydrogen penetration through those layers into the underlying metallic compounds. Recent research has demonstrated that common impurities (e.g., CO, CO2, O2) in the hydrogen stream inhibit the initial hydrogenation process but do not prevent it completely. Even at relatively high impurity concentrations, localized hydride “patches” may form, which in some instances are sufficiently large to disrupt the oxide overlayer. Consequently, during the hydrogenation process hydrogen may interact with two distinct surface types: the native oxide of the metallic compound and the hydride patches formed during the initial hydrogenation stage. This study investigates the impact of gaseous impurities on hydrogen–hydride surface interactions through both experimental and theoretical approaches. Density Functional Theory (DFT) calculations were employed to evaluate the molecular adsorption energies and interaction energies between hydrogen and impurities on the hydride surface. Application of the Langmüir model, incorporating calculated adsorption energies at various pressures and temperatures, indicates complete impurity coverage of the hydride surface, even at very low impurity concentrations. This suggests that, when the hydrogen gas phase contains such impurities, the essential H2 dissociation step is likely to be inhibited on the regular GdH2 surface. Furthermore, if dissociation occurs (at surface defects or on the coexisting oxide surface near the hydride), the penetration of the hydridic moiety (H–δ) through the hydride is generally suppressed due to its capture by impurities, forming new adsorbed species such as ·H2CO, ·HCO2, and ·OH. The results of these calculations were experimentally compared. The influence of the above impurities on hydrogenation kinetics was studied using gravimetric analysis combined with X-ray diffraction (XRD) measurements. The results corroborate with the theoretical findings, demonstrating that pure hydrogen yields the highest formation rate, while the presence of low impurity concentrations in the hydrogen stream suppresses and limits the progress of the hydrogenation process on the dihydride surface. The elucidated hydrogenation mechanisms of the impurity effect on the gadolinium hydride development provide valuable insights for the deployment of novel hydrogen storage procedures.
氢中的气相杂质:与氢化钆表面相互作用的DFT计算和实验分析
气态氢和金属化合物之间的相互作用具有重要的基础科学意义,在各种实际应用中也很重要,包括金属氢化物的形成、储能系统和催化。通常,初始氢化过程包括在天然氧化层上的表面氢解离,然后原子氢穿过这些层进入下面的金属化合物。最近的研究表明,氢流中的常见杂质(如CO, CO2, O2)抑制了初始加氢过程,但不能完全阻止它。即使在相对较高的杂质浓度下,也可能形成局部的氢化物“斑块”,在某些情况下,这些“斑块”大到足以破坏氧化层。因此,在加氢过程中,氢可以与两种不同的表面类型相互作用:金属化合物的天然氧化物和初始加氢阶段形成的氢化物斑块。本研究从实验和理论两方面探讨了气态杂质对氢化物表面相互作用的影响。采用密度泛函理论(DFT)计算氢化物表面的分子吸附能和氢与杂质的相互作用能。langm ir模型的应用,结合在不同压力和温度下计算的吸附能,表明即使在杂质浓度很低的情况下,氢化物表面也完全覆盖了杂质。这表明,当氢气相中含有此类杂质时,在规则的GdH2表面上,必需的H2解离步骤可能被抑制。此外,如果发生离解(在表面缺陷处或在氢化物附近共存的氧化物表面),氢化物部分(H -δ)通过氢化物的渗透通常由于杂质的捕获而被抑制,形成新的吸附物质,如·H2CO,·HCO2和·OH。对这些计算结果进行了实验比较。采用重量分析与x射线衍射(XRD)相结合的方法研究了上述杂质对加氢动力学的影响。结果与理论结果一致,表明纯氢的生成速率最高,而氢流中低杂质浓度的存在抑制和限制了二氢化物表面加氢过程的进展。阐明了杂质对氢化钆形成的加氢机理,为开发新型储氢工艺提供了有价值的见解。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

The Journal of Physical Chemistry C
化学-材料科学:综合
CiteScore
6.50
自引率
8.10%
发文量
2047
审稿时长
1.8 months
期刊介绍:
The Journal of Physical Chemistry A/B/C is devoted to reporting new and original experimental and theoretical basic research of interest to physical chemists, biophysical chemists, and chemical physicists.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


