Revealing Lithium Ion Transport Mechanisms and Solvation Structures in Carbonate Electrolytes
IF 14.4
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
Optimizing lithium-ion battery (LIB) electrolytes is essential for high-current applications such as electric vehicles, yet experimental techniques to characterize the complex structural dynamics responsible for the lithium transport within these electrolytes are limited. In this study, we used ultrafast infrared spectroscopy to measure chemical exchange, spectral diffusion, and solvation structures across a wide range of lithium concentrations in propylene carbonate-based LiTFSI (lithium bis(trifluoromethanesulfonimide) electrolytes, with the CN stretch of phenyl selenocyanate as the long-lived vibrational probe. Phenyl selenocyanate is shown to be an excellent dynamical surrogate for propylene carbonate in Li+ solvation clusters. A strong correlation between exchange times and ionic conductivity was observed. This correlation and other observations suggest structural diffusion as the primary transport mechanism rather than vehicular diffusion. Additionally, spectral diffusion observables measured by the probe were directly linked to the desolvation dynamics of the Li+ clusters, as supported by density functional theory and molecular dynamics simulations. These findings provide detailed molecular-level insights into LIB electrolytes’ transport dynamics and solvation structures, offering rational design pathways to advanced electrolytes for next-generation LIBs.
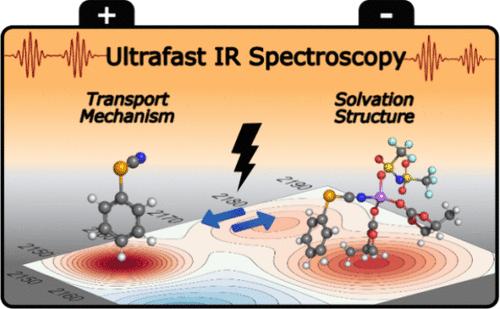
碳酸盐岩电解质中锂离子输运机制和溶剂化结构的揭示
优化锂离子电池(LIB)电解质对于电动汽车等大电流应用至关重要,但表征锂在这些电解质中传输的复杂结构动力学的实验技术有限。在这项研究中,我们使用超快红外光谱来测量碳酸丙烯基LiTFSI(锂二(三氟甲烷磺酰亚胺))电解质中不同锂浓度下的化学交换、光谱扩散和溶剂化结构,以硒氰酸苯的CN延伸作为长寿命振动探针。硒氰酸苯酯被证明是Li+溶剂化团簇中碳酸丙烯的一个很好的动态替代物。在交换时间和离子电导率之间观察到很强的相关性。这种相关性和其他观察结果表明,结构扩散是主要的运输机制,而不是车辆扩散。此外,探针测量的光谱扩散观测结果与Li+团簇的脱溶动力学直接相关,这得到了密度泛函理论和分子动力学模拟的支持。这些发现为锂离子电池电解质的传输动力学和溶剂化结构提供了详细的分子水平见解,为下一代锂离子电池的先进电解质提供了合理的设计途径。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
24.40
自引率
6.00%
发文量
2398
审稿时长
1.6 months
期刊介绍:
The flagship journal of the American Chemical Society, known as the Journal of the American Chemical Society (JACS), has been a prestigious publication since its establishment in 1879. It holds a preeminent position in the field of chemistry and related interdisciplinary sciences. JACS is committed to disseminating cutting-edge research papers, covering a wide range of topics, and encompasses approximately 19,000 pages of Articles, Communications, and Perspectives annually. With a weekly publication frequency, JACS plays a vital role in advancing the field of chemistry by providing essential research.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


