Acid Catalysis Mediated by Aqueous Hydronium Ions Formed by Contacting Zeolite Crystals with Liquid Water
IF 14.4
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
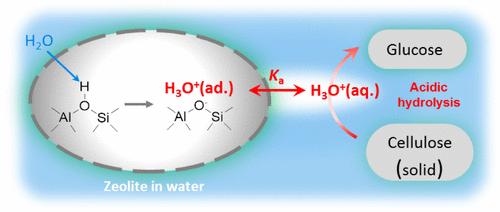
Zeolites are crystalline microporous aluminosilicates widely used as solid acids in catalytic routes to clean and sustainable energy carriers and chemicals from biogenic and fossil feedstocks. This study addresses how zeolites act as weak polyprotic acids and dissociate to form extra-crystalline hydronium (H3O+) ions in liquid water. The extent of their dissociation depends on the energy required to form the conjugate framework anions, which becomes unfavorable as the extent of dissociation increases intracrystalline charge densities because repulsive interactions ultimately preclude the detachment of all protons as catalytically relevant H3O+(aq) ions. The extent of dissociation is accurately described using electrostatic repulsion formalisms that account for aqueous H3O+ concentrations for all zeolite concentrations, Al densities, and frameworks. Probed by hydrolysis of cellulose, the most abundant biogenic polymer, this study demonstrates that zeolites catalyze this reaction exclusively through the formation of the extra-crystalline H3O+ ions at rates strictly proportional to their concentrations in the aqueous phase, irrespective of their provenance from zeolites differing in framework structure or Al content, without the purported involvement of acid sites at extracrystalline surfaces or intervening formation of smaller cellulose oligomers. The results and mechanistic interpretations seamlessly and rigorously bridge the chemistry of solid and liquid acids in aqueous media, while resolving the enduring puzzle of solid acids that catalyze transformations of substrates that cannot enter the voids where acid sites reside.
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
24.40
自引率
6.00%
发文量
2398
审稿时长
1.6 months
期刊介绍:
The flagship journal of the American Chemical Society, known as the Journal of the American Chemical Society (JACS), has been a prestigious publication since its establishment in 1879. It holds a preeminent position in the field of chemistry and related interdisciplinary sciences. JACS is committed to disseminating cutting-edge research papers, covering a wide range of topics, and encompasses approximately 19,000 pages of Articles, Communications, and Perspectives annually. With a weekly publication frequency, JACS plays a vital role in advancing the field of chemistry by providing essential research.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


