Unveiling Tetrafluoromethane Decomposition over Alumina Catalysts
IF 15.6
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
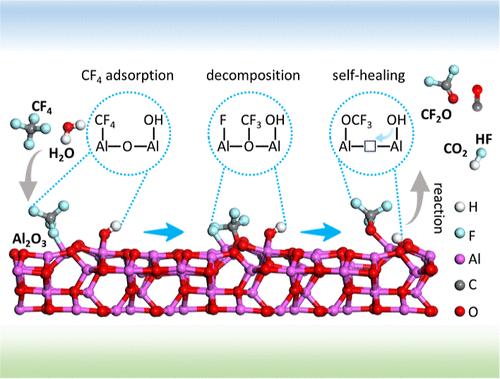
Tetrafluoromethane (CF4), the simplest perfluorocompound known as a “forever chemical”, presents substantial environmental challenges due to its health risks and contribution to the greenhouse effect. Designing efficient catalysts for CF4 decomposition remains difficult, compounded by limited understanding of the mechanisms. Here, we use constrained ab initio molecular dynamics (cAIMD) simulations and in situ experiments to elucidate the mechanism of alumina-catalyzed CF4 decomposition, highlighting the pivotal role of surface hydroxyl groups. The initial C–F bond breaking is the rate-determining step, with surface hydroxyl groups reducing the reaction free energy from 1.69 to 1.34 eV. These hydroxyl groups also facilitate the self-healing of oxygen vacancies generated during the decomposition. Contrary to the belief that CF4 decomposes directly into CO2, our cAIMD simulations, supported by synchrotron vacuum ultraviolet photoionization mass spectrometry data, reveal significant CF2O and CO byproducts. Experimental data in an anhydrous environment indicate that water primarily replenishes surface hydroxyl groups rather than directly participating in decomposition. We conclude that the relatively high efficiency of Al2O3 catalysts stems from three key properties: (1) the presence of active sites with a specific affinity for CF4 adsorption, ensuring efficient substrate interaction; (2) appropriate metal–oxygen bond strength, enabling the participation of lattice oxygen in the reaction; and (3) a high density of surface hydroxyl groups that facilitate the initial C–F bond cleavage and the self-healing of oxygen vacancies.
揭示氧化铝催化剂上的四氟甲烷分解
四氟甲烷(CF4)是被称为“永远的化学品”的最简单的全氟化合物,由于其健康风险和对温室效应的影响,给环境带来了重大挑战。设计高效的CF4分解催化剂仍然很困难,而且对机理的了解也很有限。本文采用约束从头算分子动力学(cAIMD)模拟和原位实验来阐明氧化铝催化CF4分解的机理,强调了表面羟基的关键作用。初始的C-F键断裂是反应速率的决定步骤,表面羟基使反应自由能从1.69 eV降低到1.34 eV。这些羟基还有助于分解过程中产生的氧空位的自我修复。与CF4直接分解为CO2的观点相反,我们的cAIMD模拟,在同步加速器真空紫外光电离质谱数据的支持下,揭示了大量的CF2O和CO副产物。在无水环境下的实验数据表明,水主要补充表面羟基,而不是直接参与分解。我们得出结论,Al2O3催化剂的相对高效源于三个关键性质:(1)存在对CF4吸附具有特定亲和力的活性位点,确保了高效的底物相互作用;(2)适宜的金属-氧键强度,使晶格氧参与反应;(3)表面高密度的羟基有助于初始C-F键的断裂和氧空位的自愈。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
24.40
自引率
6.00%
发文量
2398
审稿时长
1.6 months
期刊介绍:
The flagship journal of the American Chemical Society, known as the Journal of the American Chemical Society (JACS), has been a prestigious publication since its establishment in 1879. It holds a preeminent position in the field of chemistry and related interdisciplinary sciences. JACS is committed to disseminating cutting-edge research papers, covering a wide range of topics, and encompasses approximately 19,000 pages of Articles, Communications, and Perspectives annually. With a weekly publication frequency, JACS plays a vital role in advancing the field of chemistry by providing essential research.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


