Alkene Carboxy-Alkylation via CO2•–
IF 14.4
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
Herein, we introduce a new platform for alkene carboxy-alkylation. This reaction is designed around CO2•– addition to alkenes followed by radical polar crossover, which enables alkylation through carbanion attack on carbonyl electrophiles. We discovered that CO2•– adds to alkenes faster than it reduces carbonyl electrophiles and that this reactivity can be exploited by accessing CO2•– via hydrogen atom transfer from formate. This photocatalytic system transforms vinylarenes and carbonyl compounds into a diverse array of substituted γ-lactone products. Furthermore, indoles can be engaged through dearomative carboxy-alkylation, delivering medicinally relevant C(sp3)-rich heterocyclic scaffolds. Mechanistic studies reveal that the active photocatalyst is generated in situ through a photochemically induced reaction between the precatalyst and DMSO. Overall, we have developed a three-component alkene carboxy-alkylation reaction enabled by the use of formate as the CO2•– precursor.
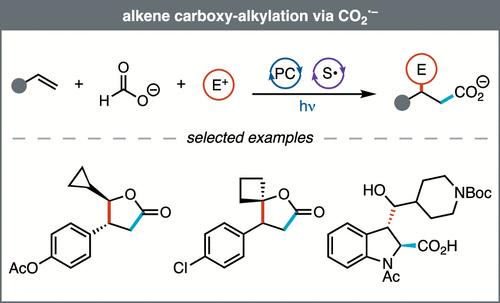
烯烃通过CO2•-羧基烷基化
本文介绍了一种新的烯烃羧基烷基化反应平台。这个反应是围绕二氧化碳•加成烯烃,然后是自由基极性交叉,这使得烷基化通过碳离子攻击羰基亲电试剂。我们发现CO2•-加入烯烃的速度比羰基亲电试剂的速度要快,这种反应性可以通过甲酸酯中的氢原子转移来获得CO2•-。这种光催化系统将乙烯基芳烃和羰基化合物转化为各种取代的γ-内酯产品。此外,吲哚可以通过脱芳羧基烷基化作用,提供具有药用价值的富含C(sp3)的杂环支架。机理研究表明,活性光催化剂是通过预催化剂与DMSO之间的光化学诱导反应在原位生成的。总之,我们开发了一种以甲酸酯为CO2•前驱体的三组分烯烃羧基烷基化反应。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
24.40
自引率
6.00%
发文量
2398
审稿时长
1.6 months
期刊介绍:
The flagship journal of the American Chemical Society, known as the Journal of the American Chemical Society (JACS), has been a prestigious publication since its establishment in 1879. It holds a preeminent position in the field of chemistry and related interdisciplinary sciences. JACS is committed to disseminating cutting-edge research papers, covering a wide range of topics, and encompasses approximately 19,000 pages of Articles, Communications, and Perspectives annually. With a weekly publication frequency, JACS plays a vital role in advancing the field of chemistry by providing essential research.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


