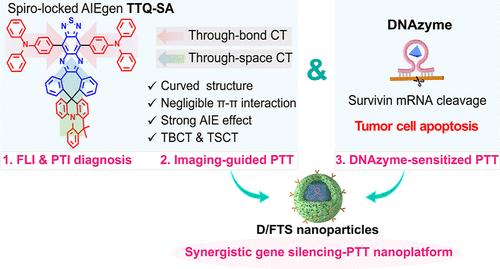
Engineering a Near-Infrared Spiro-Based Aggregation-Induced Emission Luminogen for DNAzyme-Sensitized Photothermal Therapy with High Efficiency and Accuracy
IF 14.4
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
Aggregation-induced emission luminogen (AIEgens)-based photothermal therapy (PTT) has grown into a sparkling frontier for tumor ablation. However, challenges remain due to the uncoordinated photoluminescence (PL) and photothermal properties of classical AIEgens, along with hyperthermia-induced antiapoptotic responses in tumor cells, hindering satisfactory therapeutic outcomes. Herein, a near-infrared (NIR) spiro-AIEgen TTQ-SA was designed for boosted PTT by auxiliary DNAzyme-regulated tumor cell sensitization. TTQ-SA with a unique molecular structure and packing mode was initially fabricated, endowing it with a strong AIE effect, favorable PL quantum yield, and good photothermal performance. DNAzyme, as a gene silencing tool, could alleviate antiapoptosis response during PTT. By integrating TTQ-SA and DNAzyme into folate-modified poly(lactic-co-glycolic acid) (PLGA) polymer, the as-fabricated nanosystem could promote cell apoptosis and sensitize tumor cells to PTT, thereby maximizing the therapeutic outcomes. With the combination of spiro-AIEgen-based PTT and DNAzyme-based gene silencing, the as-designed nanosystem showed promising NIR and photothermal imaging abilities for tumor targeting and demonstrated significant cell apoptotic, antitumor, and antimetastasis effects against orthotopic breast cancer. Furthermore, a synergistic antitumor effect was realized in spontaneous MMTV-PyMT transgenic mice. These findings offer new insights into AIEgen-based photothermal theranostics and DNAzyme-regulated tumor cell sensitization, paving the way for synergistic gene silencing-PTT nanoplatforms in clinical research.
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
24.40
自引率
6.00%
发文量
2398
审稿时长
1.6 months
期刊介绍:
The flagship journal of the American Chemical Society, known as the Journal of the American Chemical Society (JACS), has been a prestigious publication since its establishment in 1879. It holds a preeminent position in the field of chemistry and related interdisciplinary sciences. JACS is committed to disseminating cutting-edge research papers, covering a wide range of topics, and encompasses approximately 19,000 pages of Articles, Communications, and Perspectives annually. With a weekly publication frequency, JACS plays a vital role in advancing the field of chemistry by providing essential research.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


