Supramolecular Switching-Enabled Quorum Sensing Trap for Pathogen-Specific Recognition and Eradication to Treat Enteritis
IF 15.6
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
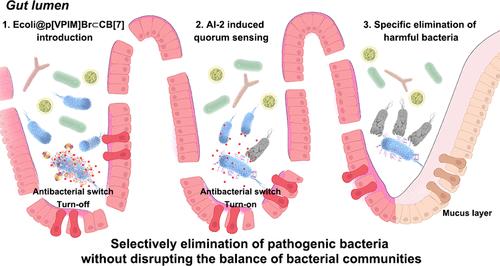
Intestinal bacterial infections have become a significant threat to human health. However, the current typical antibiotic-based therapies not only contribute to drug resistance but also disrupt gut microbiota balance, resulting in additional adverse effects on life activities. There is an urgent need to develop new antibacterial materials that selectively eliminate pathogenic bacteria without disrupting beneficial bacterial communities or promoting drug resistance. Herein, we utilize bacterial quorum sensing (QS), a universal mechanism for regulating community behavior, to develop a supramolecular QS trap by encapsulating cucurbit[7]uril (CB[7]) on 1-vinyl-3-pentylimidazolium bromide ([VPIM]Br) to form a supramolecular switch ([VPIM]Br⊂CB[7]) through host–guest interactions followed by grafting it onto bacterial cell surfaces using atom transfer radical polymerization. Subsequently, the matched pathogens are recognized and aggregated through interbacterial QS signals. Furthermore, the addition of amantadine (AD) facilitates the release of [VPIM]Br by competitive binding of CB[7] on [VPIM]Br⊂CB[7] for sterilization. This QS trap specifically triggers the self-aggregation and efficient elimination of matched bacteria. The [VPIM]Br⊂CB[7]-based trap can increase the diversity and abundance of intestinal microorganisms in mice, effectively treating Escherichia coli K88-induced intestinal damage without perturbing gut microbiota balance. This supramolecular-switched QS trap opens up a promising avenue to specifically recognize and eradicate pathogens for the antibiotic-free treatment of intestinal bacterial infections and other inflammatory diseases.
用于病原体特异性识别和根除的超分子开关群体感应陷阱治疗肠炎
肠道细菌感染已成为人类健康的重大威胁。然而,目前典型的以抗生素为基础的治疗方法不仅有助于耐药,而且还会破坏肠道菌群平衡,对生命活动产生额外的不利影响。迫切需要开发新的抗菌材料,在不破坏有益菌群或促进耐药性的情况下选择性地消灭致病菌。在这里,我们利用细菌群体感应(QS),一种调节群落行为的通用机制,通过将瓜类[7]uril (CB[7])封装在1-乙烯基-3- pentylimidazium bromide ([VPIM]Br)上,形成一个超分子开关([VPIM]Br∧CB[7]),然后利用原子转移自由基聚合将其嫁接到细菌细胞表面,从而开发出一个超分子QS陷阱。随后,匹配的病原体通过菌间QS信号被识别并聚集。此外,金刚烷胺(AD)的加入通过CB[7]在[VPIM]Br上的竞争结合来促进[VPIM]Br的释放,以达到灭菌的目的。这种QS陷阱专门触发自聚集和有效消除匹配的细菌。基于[VPIM]Br CB b[7]的诱捕器可以增加小鼠肠道微生物的多样性和丰度,在不扰乱肠道菌群平衡的情况下有效治疗大肠杆菌k88诱导的肠道损伤。这种超分子开关QS陷阱为特异性识别和根除肠道细菌感染和其他炎症性疾病的无抗生素治疗开辟了一条有前途的途径。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
24.40
自引率
6.00%
发文量
2398
审稿时长
1.6 months
期刊介绍:
The flagship journal of the American Chemical Society, known as the Journal of the American Chemical Society (JACS), has been a prestigious publication since its establishment in 1879. It holds a preeminent position in the field of chemistry and related interdisciplinary sciences. JACS is committed to disseminating cutting-edge research papers, covering a wide range of topics, and encompasses approximately 19,000 pages of Articles, Communications, and Perspectives annually. With a weekly publication frequency, JACS plays a vital role in advancing the field of chemistry by providing essential research.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


