Assembly of the Tricyclic Core of Alopecurone C by Asymmetric Donor/Donor Carbene C–H Insertion
IF 4.9
1区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
Abstract
Two routes to assemble the complete tricyclic core of alopecurone C are described. In the first-generation route, an efficient synthesis of the “eastern” half of the target, including a decagram-scale rhodium-catalyzed C–H insertion reaction, was developed. When this route proved intractable for assembling the final flavanone ring, a successful second-generation route was developed from a flavanone precursor (naringenin) employing a later stage C–H insertion. Although the second route was ultimately unsuccessful for preparation of the final target, it does provide the basis for the efficient assembly of the complete tricyclic core of alopecurone C and related flavonostilbenoid natural products.
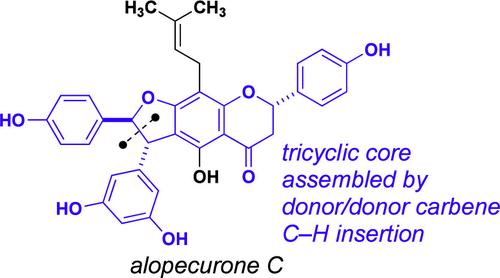
不对称给体/给体碳-氢插入对丙烯醌C三环核心的组装
介绍了两种组装全丙二烯醌三环核的方法。在第一代路线中,开发了一种高效合成目标的“东”半部分,包括十克规模的铑催化的碳氢插入反应。当这条路线被证明难以组装最终的黄酮环时,采用后期C-H插入的黄酮前体(柚皮素)开发了成功的第二代路线。虽然第二种途径最终未能成功制备最终靶点,但它确实为高效组装丙烯醌C的完整三环核心和相关的黄酮二苯乙烯类天然产物提供了基础。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Organic Letters
化学-有机化学
CiteScore
9.30
自引率
11.50%
发文量
1607
审稿时长
1.5 months
期刊介绍:
Organic Letters invites original reports of fundamental research in all branches of the theory and practice of organic, physical organic, organometallic,medicinal, and bioorganic chemistry. Organic Letters provides rapid disclosure of the key elements of significant studies that are of interest to a large portion of the organic community. In selecting manuscripts for publication, the Editors place emphasis on the originality, quality and wide interest of the work. Authors should provide enough background information to place the new disclosure in context and to justify the rapid publication format. Back-to-back Letters will be considered. Full details should be reserved for an Article, which should appear in due course.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


